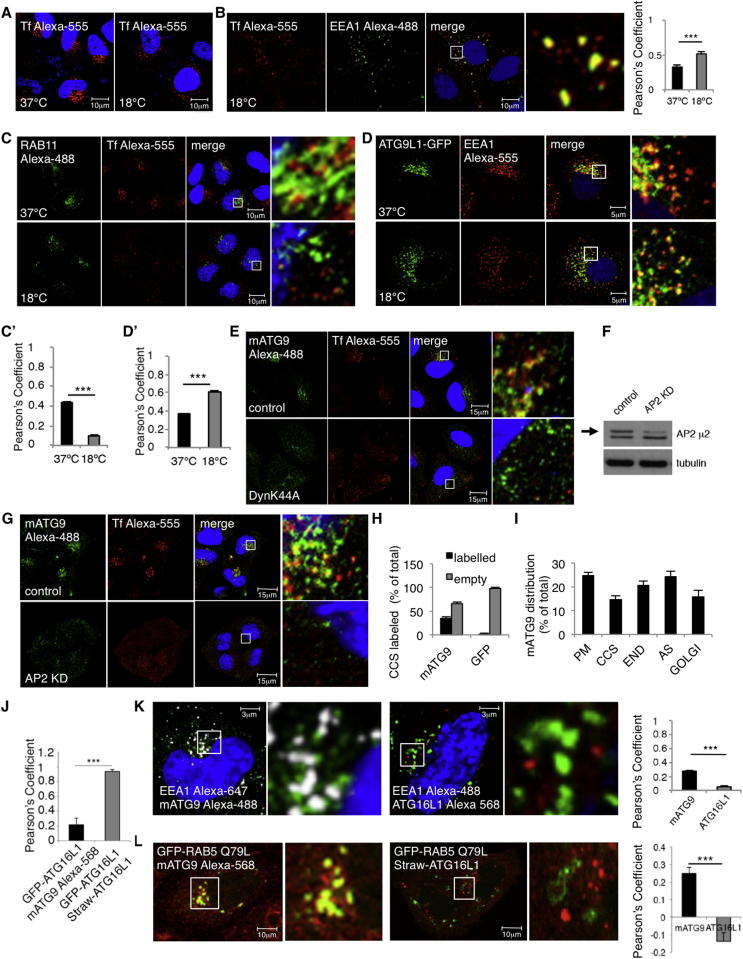
Figure S1.
mATG9 Is Trafficked from the Plasma Membrane to Early Endosomes, Related to Figure 1
(A) HeLa cells were incubated 1 hr at 18°C or 37°C and loaded for 1h with transferrin Alexa-555. The pictures represent representative fields of transferrin localization at 18°C or 37°C. Transferrin localization appears more disperse at 18°C.
(B) HeLa cells treated with the same protocol described in (A) were fixed and labeled with anti-EEA1 antibody (early endosome marker). At 18°C transferrin accumulates in early endosomes. The Pearson’s Coefficient between transferrin and EEA1 was quantified in the histogram. Error Bar = SEM. ∗∗∗ = p < 0.001.
(C and C′) HeLa cells treated as in (A) were labeled with anti-RAB11 antibody – RAB11 is the most commonly used marker for recycling endosomes. At 18°C transferrin is no longer able to reach the recycling endosome and therefore shows less colocalization with RAB11. The Pearson’s Coefficient between transferrin and RAB11 was quantified in the histogram. Error Bar = SEM. ∗∗∗ = p < 0.001.
(D and D′) HeLa cells treated as in (A) were transfected with ATG9L1-GFP and labeled with anti-EEA1- EEA1 is the most commonly used marker for early endosomes. At 18°C mATG9 is no longer able to exit from the endosome and therefore shows more colocalization with EEA1. The Pearson’s Coefficient between mATG9 and EEA1 was measured in the histogram. Error Bar = SEM. ∗∗∗ = p < 0.001.
(E) HeLa cells were transfected with dynamin dominant-negative mutant (K44A) for 20 hr, loaded for 30 min with transferrin Alexa-555 and labeled for mATG9. The pictures show a re-localization of mATG9 from perinuclear locations to a more peripheral area of the cell (close to plasma membrane).
(F and G) HeLa cells were RNA silenced for AP2 (μ2) for 5 days to inhibit clathrin-mediated endocytosis and treated as in (E). A control blot shows the level of AP2 (μ2) reduction (the arrow shows the AP2 (μ2) band).
(H) HeLa cells transfected or not with empty pEGFP vector were processed for immunogold labeling on cryosections and labeled with anti-ATG9 antibody. The GFP protein is cytoplasmic and is not associated with clathrin-coated structures. This marker was used as negative control for the specificity of mATG9 localization on clathrin-coated structures. Error bar = SEM.
(I) HeLa cells were fixed in basal conditions and processed for immunogold labeling on cryosections and stained with anti-mATG9 antibody. 10 cell profiles in two different experiments were considered by counting the number of gold particles (mATG9) in different compartments (PM: plasma membrane; CCS: clathrin-coated structures; END: endosomes; AS: autophagic structures; GOLGI: Golgi). The percentage of mATG9 localization in different compartments was quantified in the histogram and expressed as percentage of the total. Error bar = SEM.
(J) The histogram shows the quantification expressed as Pearson’s Coefficient of the experiment shown in Figure 1 G. Error bar = SEM. ∗∗∗ = p < 0.001.
(K) HeLa cells were fixed and labeled with EEA1 (early endosomes) and ATG16L1or mATG9. The Pearson’s Coefficient between EEA1 and ATG16L1 or mATG9 was quantified in the histogram. Error bar = SEM. ∗∗∗ = p < 0.001.
(L) HeLa cells were transfected with GFP-RAB5 constitutively-active mutant (Q79L) for 20 hr and mStraw-ATG16L1, or transfected with GFP-RAB5 Q79L and labeled with anti-mATG9 antibody. This constitutively-active mutant induces aberrant fusion of early endosomes. The Pearson’s Coefficient between RAB5 Q79L and mATG9 or ATG16L1 was quantified in the histogram. Error bar = SEM. ∗∗∗ = p < 0.001.