Summary
Background
Collagen-induced platelet activation is a key step in the development of arterial thrombosis via its interaction with the receptors glycoprotein (GP)VI and integrin α2β1. Adhesion and degranulation-promoting adapter protein (ADAP) regulates αIIbβ3 in platelets and αLβ2 in T cells, and is phosphorylated in GPVI-deficient platelets activated by collagen.
Objectives
To determine whether ADAP plays a role in collagen-induced platelet activation and in the regulation and function of α2β1.
Methods
Using ADAP−/− mice and synthetic collagen peptides, we investigated the role of ADAP in platelet aggregation, adhesion, spreading, thromboxane synthesis, and tyrosine phosphorylation.
Results and Conclusions
Platelet aggregation and phosphorylation of phospholipase Cγ2 induced by collagen were attenuated in ADAP−/− platelets. However, aggregation and signaling induced by collagen-related peptide (CRP), a GPVI-selective agonist, were largely unaffected. Platelet adhesion to CRP was also unaffected by ADAP deficiency. Adhesion to the α2β1-selective ligand GFOGER and to a peptide (III-04), which supports adhesion that is dependent on both GPVI and α2β1, was reduced in ADAP−/− platelets. An impedance-based label-free detection technique, which measures adhesion and spreading of platelets, indicated that, in the absence of ADAP, spreading on GFOGER was also reduced. This was confirmed with non-fluorescent differential-interference contrast microscopy, which revealed reduced filpodia formation in ADAP−/− platelets adherent to GFOGER. This indicates that ADAP plays a role in mediating platelet activation via the collagen-binding integrin α2β1. In addition, we found that ADAP−/− mice, which are mildly thrombocytopenic, have enlarged spleens as compared with wild-type animals. This may reflect increased removal of platelets from the circulation.
Keywords: ADAP, collagen, GFOGER, GPVI, integrin α2β1, platelets
Introduction
Platelet activation by collagen is a key step in the initiation of arterial thrombosis [1]. Collagen interacts directly with platelets via glycoprotein (GP)VI and integrin α2β1: GPVI is the principal collagen signaling receptor on platelets [2], and although α2β1 serves mainly as an adhesion receptor [3], several studies have suggested that it may also mediate collagen-induced signaling [4–8].
The response to collagen of GPVI-deficient platelets is weak, like that of Fc receptor γ-chain (FcRγ)-deficient (FcRγ−/−) platelets, which also lack GPVI [9,10]. We have previously shown that collagen induces tyrosine phosphorylation of adhesion and degranulation-promoting adapter protein (ADAP) in FcRγ−/− platelets[5] to a degree comparable with that induced in wild-type (WT) platelets by the GPVI-selective agonist collagen-related peptide (CRP). Phosphorylation of ADAP by collagen in WT platelets was substantially greater [5]. This suggests that, although collagen can regulate ADAP through GPVI signaling, other pathways may also be important.
ADAP is present in both T cells and platelets [11]. ADAP participates in the regulation of αLβ2 following T-cell receptor activation [12–14], in the regulation of αIIbβ3 on platelets activated by von Willebrand factor [15], and in mechanotransduction induced by shear forces exerted on platelets tethered to αIIbβ3 [16]. It has been reported that ADAP-deficient (ADAP−/−) mice have a mild hemostatic deficit [15] and mild thrombocytopenia [14]. In this study, we found that ADAP participates in collagen-induced platelet responses, mediated via both GPVI and, especially, α2β1.
Materials and methods
Materials
FcRγ−/− C57/Bl6 mice were generated by Professor Takashi Saito (Department of Molecular Genetics, Chiba University Graduate School of Medicine, Chiba, Japan) [17]; ADAP−/− C57/Bl6 mice were generated by Dr Erik J Peterson (Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA) and Professor Gary A Koretzky (University of Pennsylvania, Philadelphia, PA, USA) [14]; GPVI-deficient (GPVI−/−) C57/Bl6 mice were as previously reported [18]; WT C57/Bl6 mice were from Harlan (Bicester, UK); Ha1/29 (anti-murine α2) and Ha31/8 (anti-murine α1) antibodies were from BD Biosciences Pharmingen (Oxford, UK); fluorescein isothiocyanate (FITC)-conjugated rat antibodies against murine αIIbβ3 (Leo.H4), α2 (Sam.G4) and GPVI (JAQ1), FITC-conjugated rat IgG and non-conjugated anti-GPVI JAQ1 and Six.E10 were from EMFRET Analytics (Eibelstadt, Germany); Immulon-2HB plates were from Fisher Scientific (Loughborough, UK); Horm collagen (fibrillar, equine tendon type I) was from Axis-Shield (Dundee, UK); lotrafiban was from GlaxoSmithKline (King of Prussia, PR, USA); heparin (Monoparin) was from CP Pharmaceuticals (Wrexham, UK); other reagents were from Sigma (Poole, UK).
Murine platelet preparation
Platelets were from adult mice. Blood was drawn under terminal anesthesia into heparin (5 IU mL−1) and citrate (11 mm). Platelet-rich plasma (PRP) was obtained by centrifugation (110 × g, 5 min). PRP containing prostaglandin E1 (2 μm) was further centrifuged (2000 × g, 6 min). The platelet pellet was resuspended in Calcium Free Tyrode’s (CFT) (137 mm NaCl, 11.9 mm NaHCO3, 0.4 mm NaH2PO4, 2.7 mm KCl, 1.1 mm MgCl2, 5.6 mm glucose; pH 7.4).
Quantification of platelet glycoprotein levels
Flow cytometry was used to determine surface levels of GPVI, αIIbβ3, and α2. Five microliters of FITC-labeled antibody were incubated with 25 μL of 0.5 × 106 mL−1 washed platelets for 15 min. Platelets were diluted with 400 מL of phosphate-buffered saline, and the samples were analyzed with a Becton Dickinson FACSCalibur (Becton Dickinson, Oxford, UK).
Peptide synthesis
The synthesis of CRP, GPP, III-04 and GFOGER and the determination of melting temperatures (Tm) by polarimetry have been described previously [19–22]. Sequences and Tm values are shown in Table S1. CRP used in aggregation, thromboxane B2 (TxB2) and signaling studies was cross-linked [19].
Platelet aggregation and thromboxane assay
Turbidimetric aggregometry was carried out as previously described [23], with Bio Data PAP-4 and Chronolog aggregometers. Aggregation was monitored at 37 °C for 6 min following addition of agonist, after which thromboxane α2 (TxA2) production was measured as its stable metabolite, TxB2, with an ELISA kit (Assay Designs, Ann Arbor, MI, USA). The platelet count was 2.0 × 108 mL−1 except for aggregation studies in the presence of cangrelor and ADP, when it was 1.5 × 108 mL−1.
Signaling studies
Signaling methods have been described elsewhere [24]. Briefly, tyrosine phosphorylation of phospholipase Cγ2 (PLCγ2) and Syk was measured following immunoprecipitation by the use of SDS-PAGE and western blotting with anti-phosphotyrosine 4G10 (Millipore, Watford, UK). Protein bands on scanned images of autoradiographs were quantified with Adobe Photoshop CS. The ratio of the phosphorylation signal to the total protein signal was analyzed in a similar way to the xCELLigence data (see below).
Static platelet adhesion assay
Platelet adhesion was measured by detecting acid phosphatase in platelet lysates [25]. One hundred microliters of substrate (10 μg mL−1 in 0.01 m acetic acid) were added to 96-well plates and incubated overnight at 4 °C. Excess ligand was discarded, and the wells were blocked with 175 μL of 5% bovine serum albumin (BSA) in CFT for 1–2 h. Plates were washed three times with 175 μL per well of 0.1% BSA/CFT. Platelets were pretreated with inhibitors 15 min before addition to the wells. Preactivation with U46619 was performed immediately before addition to the plate. Platelets (1.25 × 108 mL−1, 50 μL per well) were then incubated at room temperature for 1 h. Excess platelets were discarded, and the wells were washed three times. One hundred and fifty microliters of citrate lysis buffer (3.53 mm p-nitrophenyl phosphate, 71.4 mm trisodium citrate, 28.55 mm citric acid, 0.1% [v/v] Triton X-100; pH 5.4) were added for 1 h at room temperature. One hundred microliters of 2 m NaOH were added to each well, and absorbance at 405 nm was measured with a Fluostar Optima plate reader (BMG Labtech, Aylesbury, UK). The relationship between human platelet number and absorbance has been reported previously [25]: Fig. S1 shows similar data for mouse platelets.
Real-time platelet adhesion and spreading
The xCELLigence system (Roche Diagnostics, Burgess Hill, UK) is a label-free technology that quantifies cell adhesion and spreading in real time [26,27]. It measures electrical impedance across a pair of gold-plated interdigitated microelectrodes on specialized 96-well E-plates (Fig. S2). Impedance is reported as a cell index value.
E-plates were substrate-coated as for static adhesion. Fifty microliters of CFT were added to each well and allowed to equilibrate at 37 °C, and baseline impedance measurements were recorded. Fifty microliters of washed platelets (2.5 × 108 mL−1) were then added to give a final platelet count of 1.25 × 108 mL−1. Impedance was recorded every minute for 2.5 h.
Fifty microliters of platelets (2 × 107 mL−1) were added to each well of an eight-well glass slide, coated with the indicated peptide as for the static adhesion assay. The morphology of adherent and spreading platelets was determined by wide-field differential-interference contrast microscopy with the transmitted light facility of an Olympus IX-81 FV300 confocal microscope (Olympus, Southend-on-Sea, UK) with a × 60 PlanApo objective and a numerical aperture of 1.42.
Megakaryocyte migration and apoptosis
Bone marrow was isolated from murine femora and tibiae. Megakaryocytes were cultured and purified, and migration and polyploidy analysis were carried out as described previously [28]. Surface levels of GPIb, α2 and αIIb and apoptosis were determined by flow cytometry [29].
Spleen analysis
Spleens from mice of known age, sex and weight were removed, debrided, and weighed. Spleens were fixed in 3.6% formaldehyde, mounted, and stained with hematoxylin and eosin. Megakaryocytes were quantified in each spleen by counting their number in 10 randomly selected high-power (× 60) fields.
Data analysis
Aggregation data were fitted to four-parameter logistic equations [30]. The effect of genotype on pA50 (−log EC50) values was assessed with paired t-tests and anova.
Analysis of xCELLigence data was performed on integrated cell index values (0–2.5 h). Analysis of static adhesion data was performed on absorbance values transformed as previously described [19], to minimize heteroscedasticity. Responses that were not significantly different from the negative controls, GPP and BSA, were excluded. Data were analysed by two-way anova with treatment condition as a fixed factor and experimental date as a random factor. Treatment conditions were grouped into homogeneous subsets with the Waller–Duncan test (type I/type II error ratio = 100). Conditions within a subset were considered to be not significantly different.
Logged spleen weights were analysed with ancova: sex and genotype were fixed factors; age and body weight were covariates.
Analyses were carried out with Microsoft Excel 2007 and spss 16.0 for Windows (IBM, Portsmouth, UK).
Results
Expression of GPVI, α2β1, and αIIbβ3
Flow cytometry was used to determine the levels of GPVI, α2 and αIIbβ3 on WT, ADAP−/− and FcRγ−/− platelets (Fig. S3). There were no differences in the levels of α2. The levels of GPVI were the same on WT and ADAP−/− platelets, and GPVI was undetectable on FcRγ−/− platelets. The level of αIIbβ3 was 5% lower on ADAP−/− platelets than on WT platelets (P < 0.05). The functional effect of this difference would be minor. Others have found no significant difference in αIIbβ3 levels [15].
Platelet aggregation, TxB2 production, and tyrosine phosphorylation
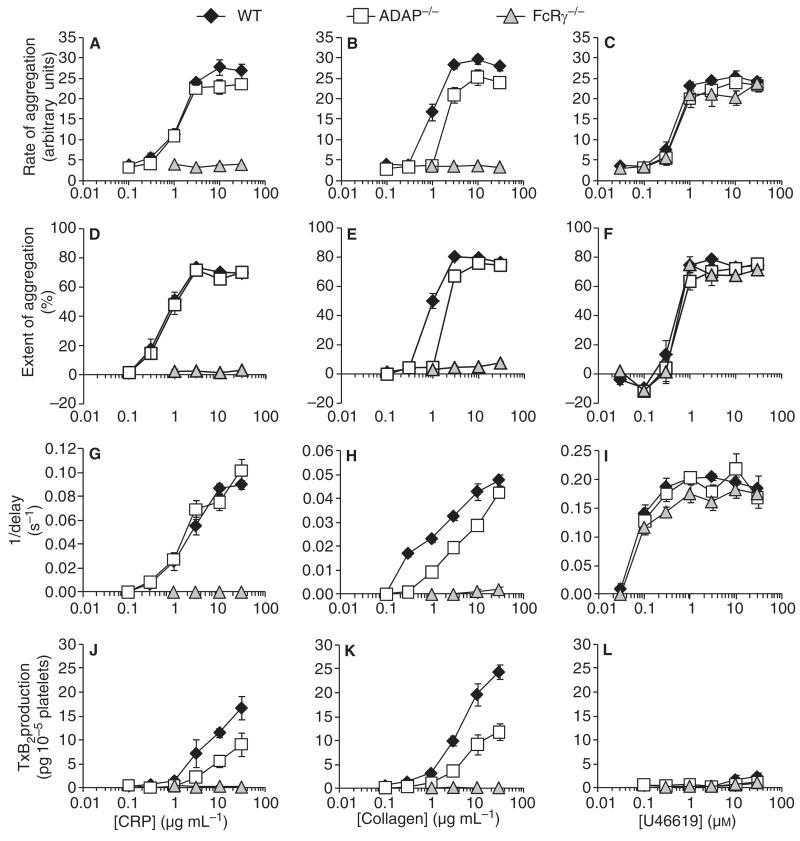
Aggregation of WT, ADAP−/− and FcRγ−/− platelets was induced by collagen, the GPVI-selective agonist CRP, and the TP receptor agonist U46619 (Fig. 1). ADAP−/− platelets responded normally to CRP and U46619; however, there was a two-fold rightward shift of the collagen concentration–response curves for rate and extent of aggregation (Table 1). The time to onset of collagen-induced responses was extended in ADAP−/− platelets. In FcRγ−/− platelets, neither CRP nor collagen induced aggregation, although U46619-induced aggregation was normal.
Fig. 1.
Aggregation of wild-type, ADAP−/− and FcRg−/− platelets and thromboxane B2 release induced by CRP, collagen and U46619. Aggregation (A–I) and thromboxane B2 (TxB2) production (J–L) induced by collagen-related peptide (CRP) (A, D, G, J) collagen (B, E, H, K) and U46619 (C, F, I, L) in wild-type (WT), adhesion and degranulation-promoting adapter protein-deficient (ADAP−/−) and Fc receptor γ-chain-deficient (FcRγ−/−) platelets. Platelet aggregation is reported as rate (A–C), extent after 6 min (D–F), and the reciprocal of the time from the addition of agonist to the onset of the response (G–I) (mean ± standard error of the mean: n = 9 for aggregation; n = 5 for TxB2 production).
Table 1.
EC50 values for the rate and extent of aggregation induced by collagen-related peptide (CRP), collagen and U46619 in wild-type (WT), adhesion and degranulation-promoting adapter protein-deficient (ADAP−/−) and Fc receptor γ-chain-deficient (FcRγ−/−) platelets
| CRP (μg mL−1) |
Collagen (μg mL−1) |
U46619 (μM) |
|
|---|---|---|---|
| Rate of aggregation: mean EC50 value (95% CI) | |||
| WT (n = 9) | 1.1 (0.78–1.6) | 1.0 (0.83–1.2) | 0.43 (0.28–0.66) |
| ADAP−/− (n = 9) | 1.4 (1.1–1.7) | 2.6 (1.7–3.9) | 0.68 (0.42–1.1) |
| FcRγ−/− (n = 5) | NR | NR | 0.51 (0.27–0.94) |
| P-value | 0.21* | < 0.0001* | 0.06† |
| Extent of aggregation: mean EC50 value (95% CI) | |||
| WT (n = 9) | 0.63 (0.39–1.0) | 0.89 (0.78–1.0) | 0.38 (0.25–0.57) |
| ADAP−/− (n = 9) | 0.69 (0.44–1.1) | 2.1 (1.8–2.5) | 0.58 (0.37–0.93) |
| FcRc−/− (n = 5) | NR | NR | 0.45 (0.29–0.68) |
| P-value | 0.71* | < 0.00001* | 0.01† |
CI, confidence interval; NR, not recorded. Statistical comparison of pA50 values (− log EC50) was carried out with paired
t-tests
or anova.
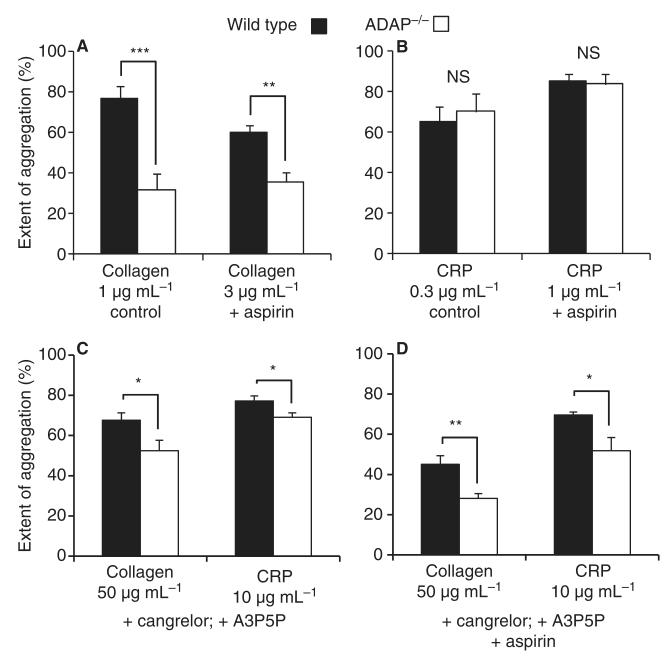
TxB2 levels were determined 6 min after addition of agonist in the aggregation samples (Fig. 1). CRP-induced and collagen-induced TxB2 production were reduced by approximately 50% in ADAP−/− as compared with WT platelets, and were undetectable in FcRγ−/− platelets. U46619 induced no detectable TxB2 production. We investigated the possibility that reduced TxA2 synthesis was responsible for the reduction in collagen-induced aggregation in ADAP−/− platelets. In the presence of aspirin (100 μm), there remained a significant reduction in collagen-induced aggregation in ADAP−/− platelets (P < 0.01). CRP-induced aggregation was the same in WT and ADAP−/− platelets in the absence and presence of aspirin (Fig. 2A,B).
Fig. 2.
Effect of aspirin, cangrelor and A3P5P on the aggregation of WT and ADAP−/− platelets induced by CRP and collagen. (A, B) The effect of aspirin (100 μm) on collagen-induced and collagen-related peptide (CRP)-induced aggregation in wild-type (WT) and adhesion and degranulation-promoting adapter protein-deficient (ADAP−/−) platelets (2 × 108 mL−1). (C, D) The effects of cangrelor (1 μm), A3P5P (1 mm) and aspirin (100 μm) on collagen-induced (50 μg mL−1) and CRP-induced (10 μg mL−1) aggregation in WT and ADAP−/− platelets (1.5 × 108 mL−1). The extent of aggregation after 6 min is shown (mean ± standard error of the mean: n = 5–7). *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant.
We further examined the effect of ADAP deficiency in the absence of the influence of released ADP and TxA2. ADP was inhibited with cangrelor (P2Y12 receptor antagonist, 1 μm) and adenosine 3′,5′-diphosphate (A3P5P: P2Y1 receptor antagonist, 1 mm). TxA2 synthesis was blocked with aspirin (100 μm). A lower platelet count (1.5 × 108 mL−1) and higher concentrations of collagen (50 μg mL−1) and CRP (10 μg mL−1) were used to overcome the effects of the inhibitors. The response to collagen was significantly lower in ADAP−/− platelets. There was also a significant effect on CRP, although this was proportionately smaller than for collagen (Fig. 2C,D).
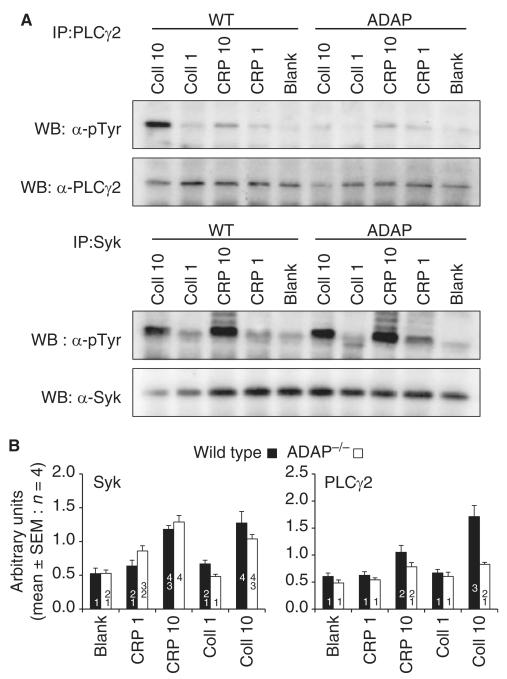
CRP-induced and collagen-induced tyrosine phosphorylation of Syk and PLCγ2 were measured in WT and ADAP−/− platelets (Fig. 3). There was no change in the phosphorylation of Syk in ADAP−/− platelets. Phosphorylation of PLCγ2 induced by collagen (10 μg mL−1) was substantially attenuated in ADAP−/− platelets. A small reduction in PLCγ2 phosphorylation induced by CRP (10 μg mL−1) was not statistically significant (Fig. 3B).
Fig. 3.
Tyrosine phosphorylation of phospholipase Cγ2 (PLCγ2) and Syk in wild-type (WT) and adhesion and degranulation-promoting adapter protein-deficient (ADAP−/−) platelets stimulated with collagen or collagen-related peptide (CRP) at 1 or 10 μg mL−1 for 6 min at 37 °C. (A) PLCγ2 and Syk were immunoprecipitated and separated by SDS-PAGE. (B) Signal quantification by densitometry (mean ± standard error of the mean [SEM]: n = 4) of phosphorylation divided by total protein. Statistical analysis was performed by anova with the Waller–Duncan post hoc test. Homogeneous subsets are indicated. Data within the same subset are not significantly different from each other. Syk phosphorylation was unchanged in ADAP−/− platelets. PLCγ2 phosphorylation induced by 10 μg mL−1 collagen was significantly attenuated in ADAP−/− platelets. Coll, collagen; IP, immunoprecipitation; WB, western blot.
Static adhesion of ADAP−/− and FcRγ−/− platelets
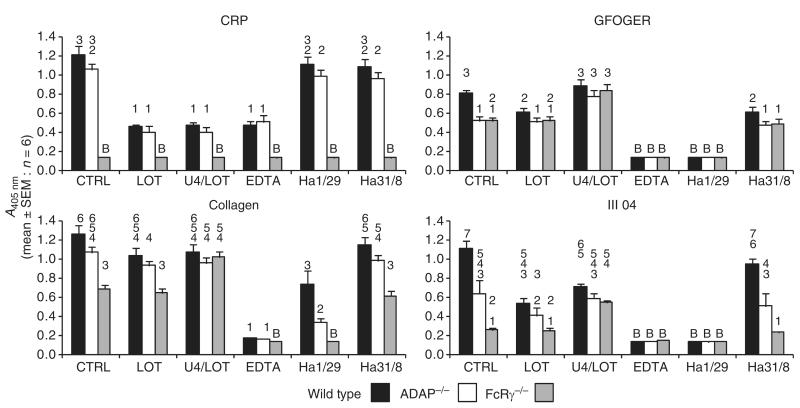
Platelet adhesion is dependent on direct interactions with ligands and secondary activation processes. These processes were investigated with collagen, CRP, GFOGER (α2β1-selective ligand [22]), and III-04, a peptide that supports α2β1-dependent and GPVI-dependent adhesion [19] (Fig. 4). Adhesion to BSA and GPP (data not shown) was indistinguishable from that of FcRγ−/− platelets to CRP.
Fig. 4.
Static adhesion of wild-type, adhesion and degranulation-promoting adapter protein-deficient (ADAP−/−) and Fc receptor γ-chain-deficient (FcRγ−/−) platelets to collagen-related peptide (CRP), GFOGER, collagen, or III-04. Platelets were either untreated (control [CTRL]) or pretreated with lotrafiban (LOT: 10 μm), U46619 (U4: 2 μm), EDTA (2 mm), anti-α2 Ha1/29 (2 μg mL) ), or anti-α1 Ha31/8 (2 μg mL−1). Platelets incubated in wells coated with bovine serum albumin (BSA) or GPP showed absorbance levels equal to those of FcRγ−/− platelets on CRP. Data labeled ‘B’ are not significantly different from responses on BSA/GPP. These data were omitted from subsequent statistical analysis. The remaining data were transformed to equalize the variance, and analyzed with anova and the Waller–Duncan post hoc test. For each peptide, homogeneous subsets are indicated. Data within the same subset are not significantly different from each other. SEM, standard error of the mean.
Adhesion to CRP was unaffected by α2-blocking or α1-blocking antibodies, but was reduced by lotrafiban (αIIbβ3 antagonist) and EDTA. There was no significant effect of ADAP deficiency on platelet adhesion to CRP. Preactivation of lotrafiban-treated platelets with U46619 did not increase binding to CRP.
Adhesion to GFOGER was significantly reduced in both ADAP−/− and FcRγ−/− platelets. Lotrafiban reduced adhesion of WT but not ADAP−/− or FcRγ−/− platelets, suggesting that the greater binding of the WT platelets was attributable to αIIbβ3-mediated interactions. Preactivation of lotrafiban-treated platelets with U46619 increased binding of WT, ADAP−/− and FcRγ−/− platelets to similar levels as that of control WT platelets, indicating that ADAP and FcRγ participate in activatory signaling following adhesion to GFOGER.
Adhesion of FcRγ−/− platelets to III-04 was substantially reduced. Residual binding was blocked by the α2-blocking antibody, showing that adhesion to III-04 is dependent on both GPVI and α2β1. Adhesion of ADAP−/− platelets was also reduced. Lotrafiban attenuated binding of WT but not ADAP−/− or FcRγ−/− platelets. Adhesion of the three genotypes was the same for U46619-activated lotrafiban-treated platelets.
Adhesion to collagen was reduced in FcRγ−/− platelets. The reduction in binding of ADAP−/− platelets was not statistically significant. Lotrafiban had no significant effect on binding. Preactivation of lotrafiban-treated platelets with U46619 restored FcRγ−/− binding levels to that observed with WT and ADAP−/− platelets. In the presence of the α2-blocking antibody, ADAP deficiency further reduced adhesion as compared with WT platelets.
Static adhesion of GPVI−/− and FcRγ−/− platelets
We have previously observed reduced adhesion of FcRγ−/− platelets to GFOGER [19]. This suggests that FcRγ may contribute to α2β1 function or that GFOGER may interact with GPVI. To investigate this, we compared the adhesion of WT, GPVI−/− (which express FcRγ [18]) and FcRγ−/− (which do not express surface GPVI [9]) platelets.
WT and FcRγ−/− platelets bound as described above. GPVI−/− platelets showed a pattern of adhesion identical to that of FcRγ−/− platelets. This included partial inhibition of adhesion to GFOGER and substantial attenuation of binding to III-04 (Fig. S4). These data suggest that GFOGER interacts weakly with GPVI.
Real-time adhesion and spreading
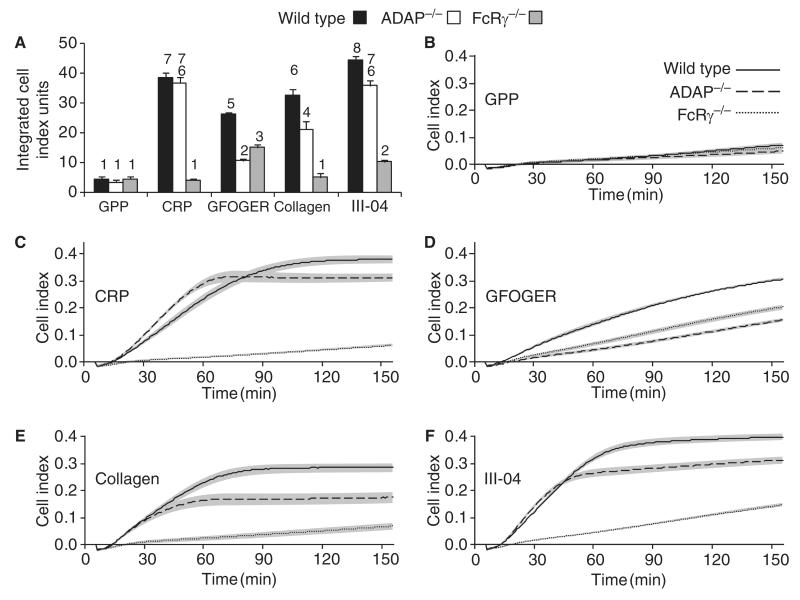
Adhesion and spreading were measured in real time for 2.5 h with the xCELLigence system. Baseline responses caused by the settling of WT, FcRγ−/− and ADAP−/− platelets on BSA (data not shown) and GPP were indistinguishable. There was a strong response on CRP with WT and ADAP−/− platelets, but only a baseline response for FcRγ−/− platelets. On collagen and III-04, the response of ADAP−/− platelets was reduced and that of FcRγ−/− platelets was substantially inhibited. On GFOGER, ADAP−/− and FcRγ−/− platelets were inhibited, although the ADAP−/− response was significantly lower than the FcRγ−/− response (Fig. 5).
Fig. 5.
Real-time adhesion and spreading of wild-type (solid line), adhesion and degranulation-promoting adapter protein-deficient (ADAP−/−) (dashed line) and Fc receptor γ-chain-deficient (FcRγ−/−) (dotted line) platelets to GPP (B), collagen-related peptide (CRP) (C), GFOGER (D), collagen (E), or III-04 (F). The xCELLigence system measured impedance every minute for 2.5 h, reported as a cell index value. Lines and shaded area = mean ± standard error of the mean: n = 6. (A) shows the area under the curve (0–2.5 h) for each condition. Statistical analysis was performed by anova with the Waller–Duncan post hoc test. Homogeneous subsets (1–8) are indicated.
We examined the morphology of WT and ADAP−/− platelets adhering to CRP and GFOGER, using video microscopy. On CRP, both genotypes bound rapidly and efficiently. Bound platelets were motile and spread actively forming lamellipodia. They also formed microaggregates. By contrast, platelets were less reactive on GFOGER and less likely to spread and form lamellipodia. They did not form microaggregates. On GFOGER, WT platelets were more active than ADAP−/− platelets, producing more filopodia (Fig. S5A–D).
Thrombocytopenia and splenomegaly in ADAP−/− mice
ADAP−/− mice had platelet levels 60–70% of those in WT and FcRγ−/− mice (Fig. S6A), consistent with previous reports of thrombocytopenia [14]. Thrombocytopenia can be associated with splenomegaly. For mice over 10 weeks old, spleens from ADAP−/− mice were 61% heavier than WT spleens and 42% heavier than FcRγ−/− spleens (Fig. S6B). Histological examination revealed increased numbers of megakaryocytes in ADAP−/− spleens as compared with WT spleens (Fig. S7). Levels of α2, αIIb and GPIb were the same in WT and ADAP−/− megakaryocytes isolated from bone marrow (data not shown). We observed no differences in ploidy, migration or levels of apoptosis between WT and ADAP−/− megakaryocytes (data not shown).
Discussion
Collagen-induced platelet activation is important in the initiation of arterial thrombosis [1], and is mediated primarily via the GPVI–FcRγ–PLCγ2 signaling pathway [2]. Integrin α2β1 supports platelet adhesion to collagen. In FcRγ−/− platelets, weak collagen-induced signaling involving ADAP [5] suggests that α2β1 mediates collagen-induced outside-in signaling. Given that ADAP contributes to the regulation of αIIbβ3 in platelets [15] and of αLβ2 in T cells [13], we hypothesized that it may also play a role in the regulation of α2β1.
Our data show that ADAP deficiency selectively attenuates collagen-induced aggregation and phosphorylation of PLCγ2. The minimal effect on CRP-induced and U46619-induced aggregation suggests that, under these experimental conditions, αIIbβ3 inside-out signaling is not substantially affected. Both collagen-induced and CRP-induced TxB2 release was attenuated, suggesting a role for ADAP in the regulation of TxA2 synthesis. Although reduced TxA2 synthesis may contribute to the inhibitory effect of ADAP deficiency on collagen-induced aggregation, inhibition was still evident in the presence of aspirin, indicating that this could be only a partial explanation. With ADP blocked, aggregation in response to both collagen and CRP is substantially attenuated [31], requiring the use of higher agonist concentrations. ADAP−/− platelets were less responsive to collagen, but also showed a slightly reduced response to CRP. This is consistent with GPVI-mediated regulation of ADAP as previously shown [5].
Both EDTA and lotrafiban reduced platelet adhesion to CRP, probably by inhibiting CRP-induced αIIbβ3-mediated platelet–platelet interactions and/or by reducing platelet spreading and hence attachment [32]. By contrast, ADAP deficiency had little effect. Hence, αIIbβ3 function was not substantially altered in the absence of ADAP under these conditions. This is consistent with the limited effect of ADAP deficiency on CRP-induced platelet aggregation.
The reduced adhesion of FcRγ−/− platelets to GFOGER and its restoration by U46619 shows that GFOGER promotes platelet activation. The similar levels of adhesion of GPVI−/− and FcRγ−/− platelets (Fig. S4) argue against a role for FcRγ in α2β1 function, as has been suggested for αIIbβ3 [33]. Alternatively, the (GPP)5 sequences flanking GFOGER may interact weakly with GPVI, this interaction being facilitated by strong binding of GFOGER to α2β1. Isolated GPVI did not bind to GFOGER but bound weakly to (GPP)10 [19], probably supported by interacting adjacent GPVI molecules. In peptides with shorter (GPP)5 flanking sequences, these interactions would not occur [34]. Hence, isolated GPVI would not bind to (GPP)5, because of the lower affinity of GPP for GpVI (as compared with GPO [34]). However, when α2β1 supports binding to GFOGER, weak interactions between GPVI and (GPP)5 might occur, inducing platelet activation. The anti-murine GPVI antibody JAQ1 [9] blocked CRP adhesion, but, contrary to expectations, increased binding of WT platelets to GFOGER. The non-blocking anti-GPVI antibody Six.E10 also increased binding to GFOGER (Fig. S8) suggesting that weak activation induced by limited antibody-induced cross-linking of GPVI can augment adhesion to GFOGER. This supports our view that weak stimuli can promote adhesion to GFOGER. By contrast, GFOGER does not induce platelet activation when applied in suspension [6,35]. Evidence that GFOGER interacts with GPVI is limited to adhesion assays conducted over long time periods.
Platelet adhesion to III-04 is strongly dependent on both GPVI and α2β1, but is also dependent on αIIbβ3 (Fig. 4). The level of adhesion of ADAP−/− platelets was intermediate between those of WT and FcRγ−/− platelets, although preactivation with U46619 revealed a uniform capacity to bind to III-04. The patterns of adhesion to GFOGER and III-04 were similar; however, FcRγ was relatively more important for adhesion to III-04. GFOGER supports better binding to α2β1 than GROGER, which is found in III-04 [20].
Adhesion to collagen was only abolished by EDTA or by the α2-blocking antibody in FcRγ−/− platelets. Adhesion of ADAP−/− platelets to collagen in the presence of the α2-blocking antibody was intermediate between that of WT and FcRγ−/− platelets. Hence, under these circumstances, a role for ADAP in adhesion mediated by GPVI and αIIbβ3 became evident.
The xCELLigence data revealed an important functional defect of ADAP−/− platelets. Responses on GFOGER were slower than on other ligands. This is consistent with observations of more extensive spreading and lamellipodia formation by murine platelets on CRP [32] than on GFOGER [6]. Both FcRγ−/− and ADAP−/− platelets had reduced responses on GFOGER, although the inhibition was greater for ADAP−/− platelets. As platelet sedimentation and total adhesion were similar, the differences in response must be attributable to the extent to which the platelets spread. These conclusions are supported by the observation of reduced filopodia formation of ADAP−/− platelets on GFOGER as compared with WT platelets (Fig. S5).
There are two ways of understanding the role of α2β1 in the platelet–collagen interaction. First, α2β1 may play an adhesive role with no outside-in signaling: activation is mediated by GPVI, and this interaction is facilitated by α2β1-mediated adhesion to collagen [36]. Blockade of α2β1 would modify signaling by disrupting the GPVI–collagen interaction, as predicted by the original two-site two-step model [37]. Second, α2β1 may also mediate signals. Blockade of α2β1 would modify platelet function by disrupting the GPVI–collagen interaction, and also by eliminating α2β1 signaling. Previous reports [4–8] and the present data support a role for α2β1 signaling. On GFOGER, ADAP−/− platelets had a lower xCELLigence response than FcRγ−/− platelets, despite similar levels of static adhesion. This indicates reduced spreading. Reduced adhesion of FcRγ−/− as compared with WT platelets is probably caused by a loss of weak GPVI signaling. As the GPVI–FcRγ pathway is functional in ADAP−/− platelets, this shows that spreading on GFOGER is mediated, at least in part, by α2β1 outside-in signaling involving ADAP.
Although selective ligands such as CRP and GFOGER are valuable, certain responses do not solely represent the nominal receptor–ligand interaction. For example, a significant component of the adhesion response to CRP is αIIbβ3-dependent. The recognition that GFOGER may induce weak activation of GPVI under certain experimental conditions has implications for the interpretation of data indicating that α2β1-mediated signaling, as induced by GFOGER, is similar to that mediated by GPVI [6].
Our observation of splenomegaly provides further information about ADAP−/− mice. The mechanisms underlying splenomegaly and thrombocytopenia remain unknown; however, it is possible that the two phenomena are connected. Furthermore, although low platelet counts alone may not account for changes in bleeding time in normal mice [38], mild thrombocytopenia [14], defects in αIIbβ3 function [15,16] and defects in collagen-induced platelet activation may all contribute to the increase in rebleeding time observed in ADAP−/− mice [15].
Kasirer-Friede et al. [15] reported limited findings on aggregation, but they did show the effect of ADAP deficiency on ADP-induced, protease-activated receptor 4 ligand-induced and convulxin-induced FITC–fibrinogen binding. However, although there was a 75% reduction in FITC–fibrinogen binding induced by 10 μm ADP, the only aggregation defect reported occurred at 2–5 μm ADP, at which concentrations aggregation was present but reversible, as compared to the wild type. These observations highlight the difficulty in comparing different techniques, as the substantial reduction in fibrinogen binding had such a minimal effect on aggregation. By contrast, our data reveal a selective effect of ADAP deficiency on collagen-induced aggregation, clearly implicating α2β1 in the platelet activation process induced by collagen.
Supplementary Material
Supporting figures 1-8
Fig. S1. Relationship between platelet number and absorbance values obtained in the static adhesion assay.
Fig. S2. Photograph of the microelectrode array on the base of an xCELLigence E-plate well.
Fig. S3. Expression levels of GPVI, α2 and αIIbβ3.
Fig. S4. Static adhesion of WT, GPVI−/− and FcRγ−/− platelets to CRP, GFOGER, collagen or III-04.
Fig. S5. Videos of WT (A, C) and ADAP−/− (B, D) platelets adhering to CRP (A, B) and GFOGER (C, D).
Fig. S6. Thrombocytopenia and splenomegaly in ADAP−/− mice.
Fig. S7. Histological sections of WT and ADAP−/− spleens.
Fig. S8. Effect of anti-murine GPVI antibodies JAQ1 (10 μg mL−1) and Six.E10 (10 μg mL−1) on adhesion of WT mouse platelets to collagen peptides.
Supporting table 1
Table S1. Sequences and melting temperatures (Tm) of synthetic peptides.
Video 5A: WT & CRP
Video 5B: ADAP−/− & CRP
Video 5C: WT & GFOGER
Video 5D: ADAP−/− & GFOGER
Acknowledgements
We thank E. Peterson and G. Koretzky for procuring the ADAP−/− mice used in these studies, A. Mazharian for assistance with megakaryocyte analysis, and B. Grygielska for assistance with protein signaling studies. This work was supported by the Medical Research Council (G. E. Jarvis and R. W. Farndale), the British Heart Foundation (R. W. Farndale), and the Wellcome Trust (R. W. Farndale, Ref. 068724; and S.P. Watson, Ref. 088410).
Footnotes
Disclosure of Conflict of Interests The authors state that they have no conflict of interest.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Farndale RW, Sixma JJ, Barnes MJ, De Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2:561–73. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 2.Nieswandt B, Watson SP. Platelet–collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–61. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 3.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–12. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 4.Ichinohe T, Takayama H, Ezumi Y, Arai M, Yamamoto N, Takahashi H, Okuma M. Collagen-stimulated activation of Syk but not c-Src is severely compromised in human platelets lacking membrane glycoprotein VI. J Biol Chem. 1997;272:63–8. doi: 10.1074/jbc.272.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis GE, Best D, Watson SP. Glycoprotein VI/Fc receptor gamma chain-independent tyrosine phosphorylation and activation of murine platelets by collagen. Biochem J. 2004;383:581–8. doi: 10.1042/BJ20040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCγ2. J Cell Biol. 2003;160:769–80. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundaresan P, Farndale RW. P38 mitogen-activated protein kinase dephosphorylation is regulated by protein phosphatase 2A in human platelets activated by collagen. FEBS Lett. 2002;528:139–44. doi: 10.1016/s0014-5793(02)03277-5. [DOI] [PubMed] [Google Scholar]
- 8.Mazzucato M, Cozzi MR, Battiston M, Jandrot-Perrus M, Mongiat M, Marchese P, Kunicki TJ, Ruggeri ZM, De Marco L. Distinct spatio-temporal Ca2+ signaling elicited by integrin alpha2beta1 and glycoprotein VI under flow. Blood. 2009;114:2793–801. doi: 10.1182/blood-2008-12-193490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J Biol Chem. 2000;275:23998–4002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- 10.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest. 1989;84:1440–5. doi: 10.1172/JCI114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–93. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffths EK, Krawczyk C, Kong YY, Raab M, Hyduk SJ, Bouchard D, Chan VS, Kozieradzki I, Oliveira-Dos-Santos AJ, Wakeham A, Ohashi PS, Cybulsky MI, Rudd CE, Penninger JM. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293:2260–3. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Wei B, Bismuth G, Rudd CE. SLP-76-ADAP adaptor module regulates LFA-1 mediated costimulation and T cell motility. Proc Natl Acad Sci USA. 2009;106:12436–41. doi: 10.1073/pnas.0900510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu QH, Pribila JT, Freedman BD, Shimizu Y, Koretzky GA. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–5. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 15.Kasirer-Friede A, Moran B, Nagrampa-Orje J, Swanson K, Ruggeri ZM, Schraven B, Neel BG, Koretzky G, Shattil SJ. ADAP is required for normal alphaIIbbeta3 activation by VWF/GP Ib–IX–V and other agonists. Blood. 2007;109:1018–25. doi: 10.1182/blood-2006-05-022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasirer-Friede A, Ruggeri ZM, Shattil SJ. Role for ADAP in shear flow-induced platelet mechanotransduction. Blood. 2010;115:2274–82. doi: 10.1182/blood-2009-08-238238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SY, Ueda S, Ohno H, Hamano Y, Tanaka M, Shiratori T, Yamazaki T, Arase H, Arase N, Karasawa A, Sato S, Ledermann B, Kondo Y, Okumura K, Ra C, Saito T. Resistance of Fc receptor-deficient mice to fatal glomerulonephritis. J Clin Invest. 1998;102:1229–38. doi: 10.1172/JCI3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–7. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis GE, Raynal N, Langford JP, Onley DJ, Andrews A, Smethurst PA, Farndale RW. Identification of a major GpVI-binding locus in human type III collagen. Blood. 2008;111:4986–96. doi: 10.1182/blood-2007-08-108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raynal N, Hamaia SW, Siljander PR, Maddox B, Peachey AR, Fernandez R, Foley LJ, Slatter DA, Jarvis GE, Farndale RW. Use of synthetic peptides to locate novel integrin alpha2beta1-binding motifs in human collagen III. J Biol Chem. 2006;281:3821–31. doi: 10.1074/jbc.M509818200. [DOI] [PubMed] [Google Scholar]
- 21.Knight CG, Morton LF, Onley DJ, Peachey AR, Ichinohe T, Okuma M, Farndale RW, Barnes MJ. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc Res. 1999;41:450–7. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 22.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins alpha(1)-beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275:35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis GE. Platelet aggregation: turbidimetric measurements. Methods Mol Biol. 2004;272:65–76. doi: 10.1385/1-59259-782-3:065. [DOI] [PubMed] [Google Scholar]
- 24.Pearce AC, Senis YA, Billadeau DD, Turner M, Watson SP, Vigorito E. Vav1 and vav3 have critical but redundant roles in mediating platelet activation by collagen. J Biol Chem. 2004;279:53955–62. doi: 10.1074/jbc.M410355200. [DOI] [PubMed] [Google Scholar]
- 25.Onley DJ, Knight CG, Tuckwell DS, Barnes MJ, Farndale RW. Micromolar Ca2+ concentrations are essential for Mg2+-dependent binding of collagen by the integrin alpha 2beta 1 in human platelets. J Biol Chem. 2000;275:24560–4. doi: 10.1074/jbc.M004111200. [DOI] [PubMed] [Google Scholar]
- 26.Castillo-Briceno P, Bihan D, Nilges M, Hamaia S, Meseguer J, Garcia-Ayala A, Farndale RW, Mulero V. A role for specific collagen motifs during wound healing and inflammatory response of fibroblasts in the teleost fish gilthead seabream. Mol Immunol. 2011;48:826–34. doi: 10.1016/j.molimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- 28.Mazharian A, Watson SP, Severin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37:1238–49. doi: 10.1016/j.exphem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tijssen MR, Woelders H, De Vries-van Rossen A, van der Schoot CE, Voermans C, Lagerberg JW. Improved postthaw viability and in vitro functionality of peripheral blood hematopoietic progenitor cells after cryopreservation with a theoretically optimized freezing curve. Transfusion. 2008;48:893–901. doi: 10.1111/j.1537-2995.2008.01650.x. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis GE, Atkinson BT, Frampton J, Watson SP. Thrombin-induced conversion of fibrinogen to fibrin results in rapid platelet trapping which is not dependent on platelet activation or GPIb. Br J Pharmacol. 2003;138:574–83. doi: 10.1038/sj.bjp.0705095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarvis GE, Best D, Watson SP. Differential roles of integrins alpha2-beta1 and alphaIIbbeta3 in collagen and CRP-induced platelet activation. Platelets. 2004;15:303–13. doi: 10.1080/09537100410001710254. [DOI] [PubMed] [Google Scholar]
- 32.Thornber K, McCarty OJ, Watson SP, Pears CJ. Distinct but critical roles for integrin alphaIIbbeta3 in platelet lamellipodia formation on fibrinogen, collagen-related peptide and thrombin. FEBS J. 2006;273:5032–43. doi: 10.1111/j.1742-4658.2006.05500.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Ware J, Jackson CW, Gartner TK. FcRgamma-chain-dependent alphaIIbeta3 elicited outside-in signaling. J Thromb Haemost. 2007;5:426–8. doi: 10.1111/j.1538-7836.2007.02317.x. [DOI] [PubMed] [Google Scholar]
- 34.Smethurst PA, Onley DJ, Jarvis GE, O’Connor MN, Knight CG, Herr AB, Ouwehand WH, Farndale RW. Structural basis for the platelet–collagen interaction: the smallest motif within collagen that recognizes and activates platelet glycoprotein VI contains two glycine-proline-hydroxyproline triplets. J Biol Chem. 2007;282:1296–304. doi: 10.1074/jbc.M606479200. [DOI] [PubMed] [Google Scholar]
- 35.Achison M, Elton CM, Hargreaves PG, Knight CG, Barnes MJ, Farndale RW. Integrin-independent tyrosine phosphorylation of p125(fak) in human platelets stimulated by collagen. J Biol Chem. 2001;276:3167–74. doi: 10.1074/jbc.M007186200. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson BT, Jarvis GE, Watson SP. Activation of GPVI by collagen is regulated by alpha2beta1 and secondary mediators. J Thromb Haemost. 2003;1:1278–87. doi: 10.1046/j.1538-7836.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 37.Santoro SA, Walsh JJ, Staatz WD, Baranski KJ. Distinct determinants on collagen support alpha 2 beta 1 integrin-mediated platelet adhesion and platelet activation. Cell Regul. 1991;2:905–13. doi: 10.1091/mbc.2.11.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez V, Govezensky T, Gevorkian G, Larralde C. Low platelet counts alone do not cause bleeding in an experimental immune thrombocytopenic purpura in mice. Haematologica. 2003;88:679–87. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting figures 1-8
Fig. S1. Relationship between platelet number and absorbance values obtained in the static adhesion assay.
Fig. S2. Photograph of the microelectrode array on the base of an xCELLigence E-plate well.
Fig. S3. Expression levels of GPVI, α2 and αIIbβ3.
Fig. S4. Static adhesion of WT, GPVI−/− and FcRγ−/− platelets to CRP, GFOGER, collagen or III-04.
Fig. S5. Videos of WT (A, C) and ADAP−/− (B, D) platelets adhering to CRP (A, B) and GFOGER (C, D).
Fig. S6. Thrombocytopenia and splenomegaly in ADAP−/− mice.
Fig. S7. Histological sections of WT and ADAP−/− spleens.
Fig. S8. Effect of anti-murine GPVI antibodies JAQ1 (10 μg mL−1) and Six.E10 (10 μg mL−1) on adhesion of WT mouse platelets to collagen peptides.
Supporting table 1
Table S1. Sequences and melting temperatures (Tm) of synthetic peptides.
Video 5A: WT & CRP
Video 5B: ADAP−/− & CRP
Video 5C: WT & GFOGER
Video 5D: ADAP−/− & GFOGER