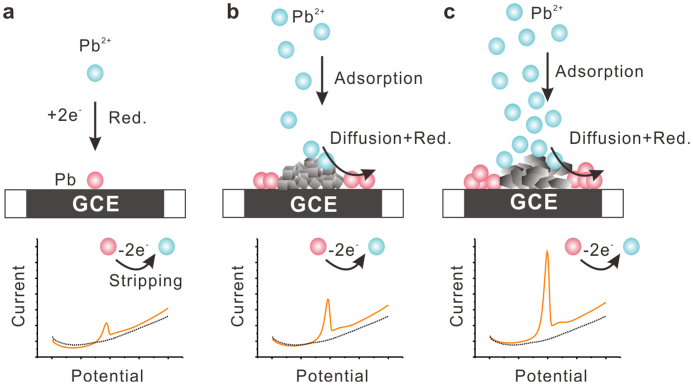
Figure 5. Schematics of how adsorptive nanoscale materials exposed with different crystal facets could be designed to enhance the performance of electrochemical sensing.
(a), Bare GCE. (b), Co3O4 nanocubes modified GCE. (c), Co3O4 nanoplates modified GCE. Facet-dependent electrochemical properties of Co3O4 nanocrystals toward heavy metal ions (e.g. Pb2+) are investigated. The (111)-bound Co3O4 nanoplates are superior to (001)-bound Co3O4 nanocubes. Adsorption measurements reveal that Co3O4 (111) facet can adsorb more metal ions than the (001) facet. DFT calculations demonstrate that Pb on Co3O4(111) facet exhibits larger adsorption energies, more adsorption sites, and faster diffusion than on (001) facet.