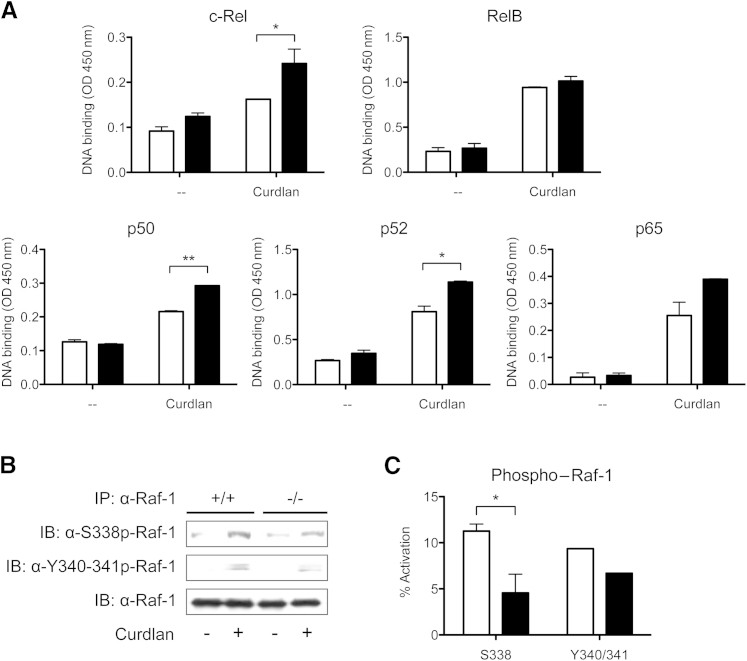
Figure 6.
Galectin-3 negatively regulates c-Rel transcription factor activation through the Raf-1 signaling pathway. A: Nuclear extracts from unstimulated or curdlan-stimulated gal3+/+ (white bars) and gal3−/− (black bars) DCs were added to plates coated with oligonucleotide containing an NF-κB consensus-binding site. Binding of activated NF-κB subunits was detected using antibodies against c-Rel, RelB, p50, p52, and p65. B: Raf-1 phosphorylation at Ser338 and Tyr340/341 as determined by immunoblot (IB) analysis in unstimulated and curdlan-stimulated gal3+/+ and gal3−/− DCs. Data are representative of at least two independent experiments. IP, immunoprecipitate. C: Densitometry analysis of phosphorylated Raf-1. Percentage of activation was calculated as the ratio of phosphorylated Raf-1 to total Raf-1. Data are given as means ± SD and are representative of two independent experiments. ∗P < 0.05, ∗∗P < 0.01.