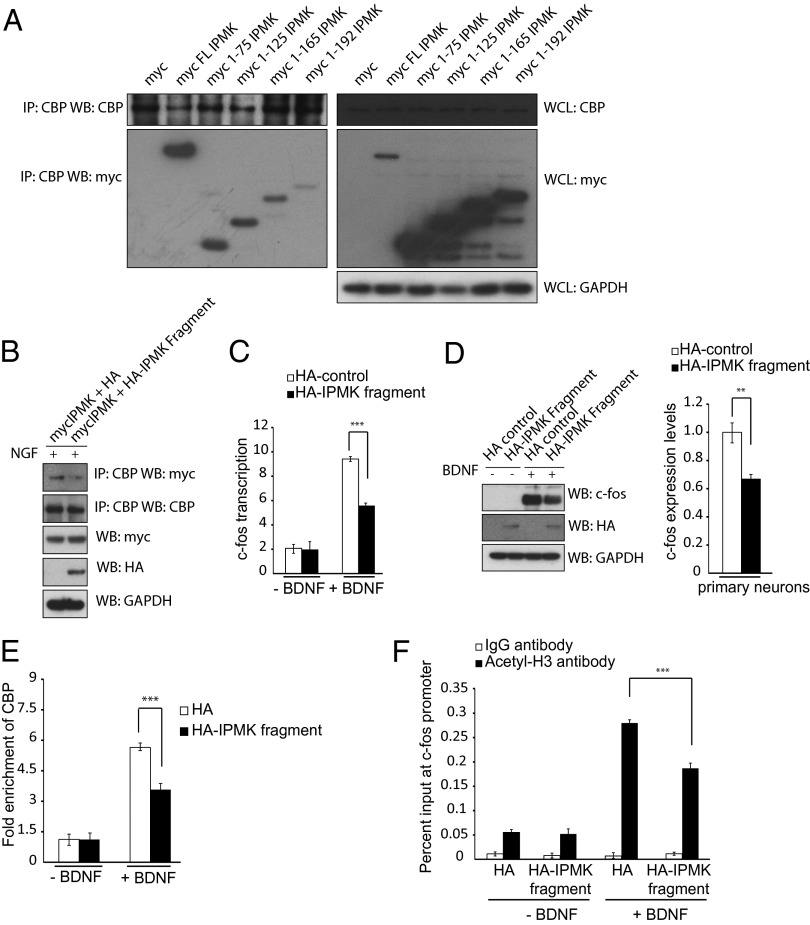
Fig. 3.
A dominant negative construct of IPMK prevents IPMK-CBP association and CBP-mediated acetylation of the c-fos promoter. (A) C-terminal deletion constructs of myc-IPMK are transfected into PC12 cells. CBP is immunoprecipitated, and the myc tag is probed via Western blot. (B) PC12 cells overexpressing a dominant-negative fragment of HA-IPMK encompassing amino acids 1–75 show decreased levels of IPMK-CBP binding. (C) Real-time PCR is used to assess c-fos transcription levels in primary cortical neurons after BDNF treatment. Neurons expressing a dominant-negative IPMK fragment show decreased transcription levels of c-fos. ***P < 0.001. Data are means ± SEM from three experiments. (D) c-fos protein levels are assayed in primary neurons expressing control HA-tag or HA-tagged IPMK dominant negative after BDNF treatment using Western blot. Neurons containing an overexpressed dominant-negative IPMK fragment show diminished c-fos protein levels. **P < 0.01. Data are means ± SEM from three experiments. (E) The ChIP assay is used to assess CBP recruitment to the c-fos promoter after BDNF treatment in neurons infected with control HA-tag or HA-tagged dominant negative construct. Cells containing the dominant-negative IPMK fragment show decreased recruitment to the promoter. ***P < 0.001. Data are means ± SEM from three experiments. (F) The ChIP assay is used to assess histone H3 acetylation at the c-fos promoter after BDNF treatment. Cells containing the dominant-negative IPMK fragment demonstrate decreased levels of histone acetylation. ***P < 0.001. Data are means ± SEM from three experiments.