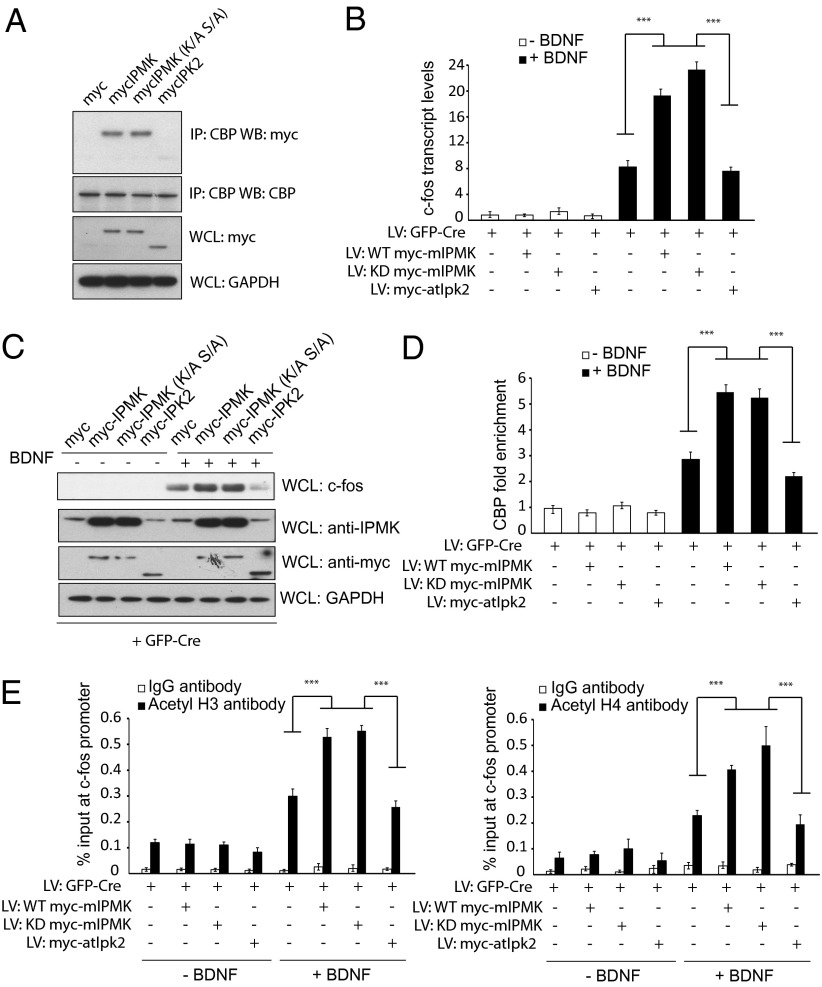
Fig. 4.
IPMK kinase activity is not required for binding to CBP. (A) Determinants of the IPMK-CBP interaction. Myc-tagged wild-type mIPMK, kinase-dead mIPMK, and atIpk2 are transfected into PC12 cells. Binding between various constructs of IPMK and CBP is assessed via coimmunoprecipitation experiments. Although wild-type mIPMK and kinase-dead mIPMK coimmunoprecipitates with CBP, atIpk2 does not. (B) Wild-type and kinase-dead IPMK, but not atIpk2, can complement IPMK deficiency in c-fos transcription. Endogenous IPMK is depleted in IPMKfl/fl neurons by infection with lentiviral particles expressing GFP-Cre. Control myc peptide, wild-type mIPMK, kinase-dead mIPMK, and atIpk2 are coexpressed using lentivirus. Neurons are stimulated with BDNF, and mRNA of c-fos was assessed via qPCR. (C) Western blotting reveals an increase in c-fos protein levels in IPMK-null neurons complemented with wild-type and kinase-dead IPMK, but not atIpk2. (D) ChIP using anti-CBP antibody reveals increased CBP recruitment to the c-fos promoter on complementation of IPMK-deleted neurons with wild-type or kinase-dead, but not atIpk2, IPMK. P < 0.001. Data are means ± SEM from three experiments. (E) Infection of IPMK-deleted neurons with wild-type or kinase-dead IPMK enhances histone H3 and H4 acetylation at the c-fos promoter after treatment with BDNF. Such an increase is not seen with expression of atIpk2 IPMK in IPMK-null neurons. P < 0.001. Data are means ± SEM from three experiments.