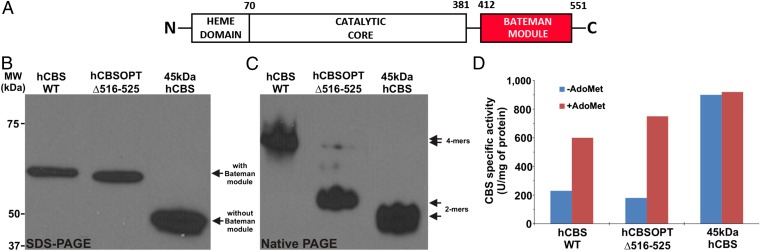
Fig. 1.
Architecture and biochemical properties of hCBSOPTΔ516–525. (A) In hCBSOPTΔ516–525, unlike the truncated 45-kDa hCBS lacking the Bateman module (red), the distribution of the functional domains is not affected as compared with the native hCBS. (B and C) SDS/PAGE (B) and native PAGE (C) Western blots of the purified enzymes probed with monoclonal anti-CBS antibody show the predominantly dimeric form of hCBSOPTΔ516–525 in contrast to native tetramers of wild-type hCBS and dimers of 45-kDa hCBS. (D) CBS activities ± 300 µM AdoMet show that our hCBSOPTΔ516–525 construct has basal activity and is activated by AdoMet to a similar extent as wild-type hCBS, unlike the constitutively activated 45-kDa hCBS.