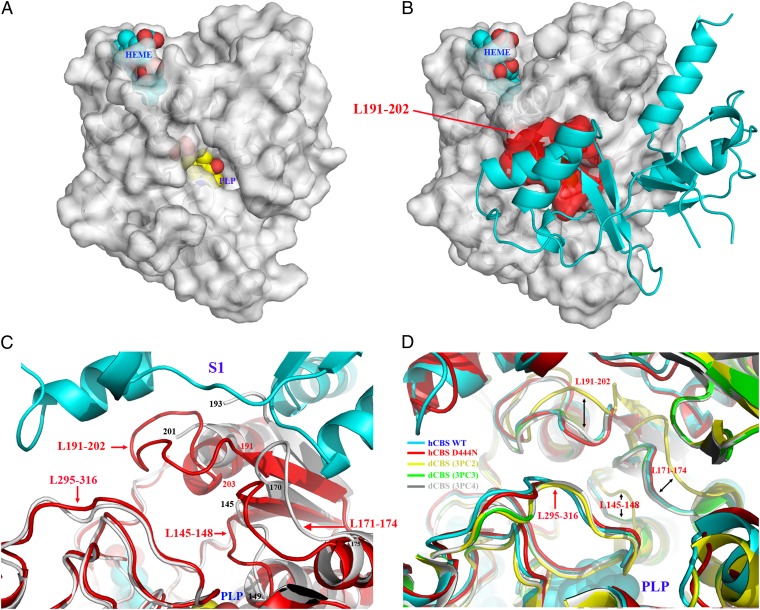
Fig. 4.
Structural elements determining access to the active site. (A) Surface representation of the 45-kDa hCBS shows that, in the absence of the regulatory domain, the catalytic cavity remains open and the PLP is exposed. (B) In contrast, in the basal form of hCBS, the entrance to the cavity is occluded by the Bateman module by the closed conformation of the loops L145–148, L171–174, and L191–202. (C) A zoom-in view of the interface region between the catalytic core and Bateman module showing the structural elements at the entrance of the catalytic site in hCBS (red) and in the 45-kDa hCBS (gray). (D) Superimposition of the region shown in C for the basal form of hCBS (cyan), for the pathogenic D444N hCBS mutant (red), and for dCBS in the absence (yellow) or the presence (green and gray, respectively) of bound substrates.