Significance
This study reports experimental evidence for the “public goods dilemma” between cooperators and cheaters in an asexual ant society, in which cheating is always more rewarding for individuals but cooperation at the cost of individual fitness leads to better performance of groups. Although this dilemma provides the basic principle of social evolution, its experimental demonstration with underlying genetics and fitness evaluation for both cooperators and cheaters still lacks in societies other than microbial ones. By showing the striking evolutionary convergence between microbial societies and insect societies in fitness consequences and in phenotypic plasticity of cooperators against cheaters, our result suggests that a wide range of scales of cooperative systems could be understood in a unified manner.
Keywords: social evolution, public goods game, social parasitism, self-organization, phenotypic plasticity
Abstract
Cooperation in biological, social, and economic groups is underpinned by public goods that are generated by group members at some personal cost. Theory predicts that public goods will be exploited by cheaters who benefit from the goods by not paying for them, thereby leading to the collapse of cooperation. This situation, described as the “public goods dilemma” in game theory, makes the ubiquity of cooperation a major evolutionary puzzle. Despite this generalization, the demonstration of genetic background and fitness effects of the public goods dilemma has been limited to interactions between viruses and between cells, and thus its relevance at higher levels of organismal complexity is still largely unexplored. Here we provide experimental evidence for the public goods dilemma in a social insect, the ant Pristomyrmex punctatus. In this species, all workers are involved in both asexual reproduction and cooperative tasks. Genetic cheaters infiltrate field colonies, reproducing more than the workers but shunning cooperative tasks. In laboratory experiments, cheaters outcompeted coexisting workers in both survival and reproduction, although a group composed only of cheaters failed to produce offspring. The operations of the public goods dilemma in P. punctatus showed a remarkable convergence with those in microbial societies, not only in fitness consequences but also in behavioral mechanisms. Our study reinforces the evolutionary impact of cheaters on diverse cooperative systems in the laboratory and in the field.
Cooperation is a hallmark of life on Earth, ranging from ancient self-replicating molecules to modern human societies, and has provided the driving force behind the major transitions in evolution (1, 2). In cooperative groups, public goods are usually generated and shared by component individuals at some personal cost. However, the publicness of the goods provides an incentive for some group members, called cheaters (defectors, exploiters, or free-riders), to benefit from the goods without contributing to them. The cheaters may ruin the public goods, or at least greatly diminish their utility. Therefore, the observed prevalence of cooperation in living systems poses a conundrum (1, 2). The expected conflict between cooperators and cheaters is well captured by the “public goods dilemma” (or “N-person prisoner’s dilemma”) in game theory. In this multiplayer social dilemma, individual players either cooperate or cheat in their contribution to the public goods. Cheating is always more rewarding for the competition within groups, whereas cooperation leads to better performance for the competition between groups. Evolutionary consequences of the public goods dilemma have been analyzed extensively using game theoretical models to study how cooperation survives cheating (3–8).
There is also a large amount of literature on experimental studies of the public goods dilemma in biological and social sciences. Classic experiments with wild animals [mainly vertebrates (9–11)] and humans [known as experimental economics (12–15)] construct artificial public goods systems so that interacting individuals confront the dilemma, and examine whether and how they cooperate (or cheat) in line with predictions of theories. A more direct test for the evolutionary significance of the public goods dilemma involves a mixture of cooperators and cheaters with known genetic backgrounds, and examines whether a population of the former is invaded by the latter (and vice versa) by measuring the fitness of both. In the past decade, experiments using systems in which viruses or single cells are the component individuals have offered a great opportunity for such a direct test. Examples include coinfecting RNA phages (16), chimeric aggregations of unicellular microbes (17−23), and cancerous multicellular organisms (24). However, no comparable experiments exist using more complex cooperative groups of multicellular individuals. Here, we provide direct experimental evidence of the public goods dilemma faced by genetic cooperators and cheaters in colonies of a social insect.
A defining characteristic of cooperation in social insects is reproductive division of labor, under which a reproductive role is limited to a subset of colony members (usually queens), with the other members (workers) concentrating on cooperative tasks. Mutant cheaters can undermine the reproductive division of labor by reproducing without helping. Indeed, such selfish behaviors have been reported in some workers of honey bees (Apis mellifera) that produce their own sons (25) or even queen-destined asexual daughters (26) in queen-right colonies, and in immature females sired by particular males of several polyandrous (queen multiple mating) species that show higher potential to develop into new queens than other patrilines (27–29). However, direct comparison of fitness between cooperators and cheaters is lacking in these cases. In addition, alternative mechanisms have been proposed in some cases that result in the same outputs (30–35).
Among social insects with known genetic cheaters is the ant Pristomyrmex punctatus. In this species, the morphologically and functionally distinct queen caste is secondarily lost; instead, all morphologically defined workers are involved in both parthenogenetic (thelytokous) reproduction and cooperative tasks (36, 37). Reproductive division of labor is realized through age polyethism (or temporal castes) among workers, where younger workers reproduce and engage in inside-nest tasks, such as brood care, and older workers cease reproduction and shift to outside-nest tasks, such as foraging (38). Cheater individuals, which constitute a single intraspecific lineage in the field (39, 40), engage in few tasks except for reproduction (41). In addition, these individuals show several characteristics that are typical in queens of other ants, such as having more ovarioles, a larger body size, and three conspicuous ocelli (39–42). Using experimental colonies with varying proportions of cheaters, we tested whether the fitness of cheaters and workers in a colony of P. punctatus conformed to the framework of the public goods dilemma. We also examined how social regulation of the colony, measured by task allocation to outside-nest activity, was subverted by cheaters.
Results
Individual Fitness of Cheaters and Workers.
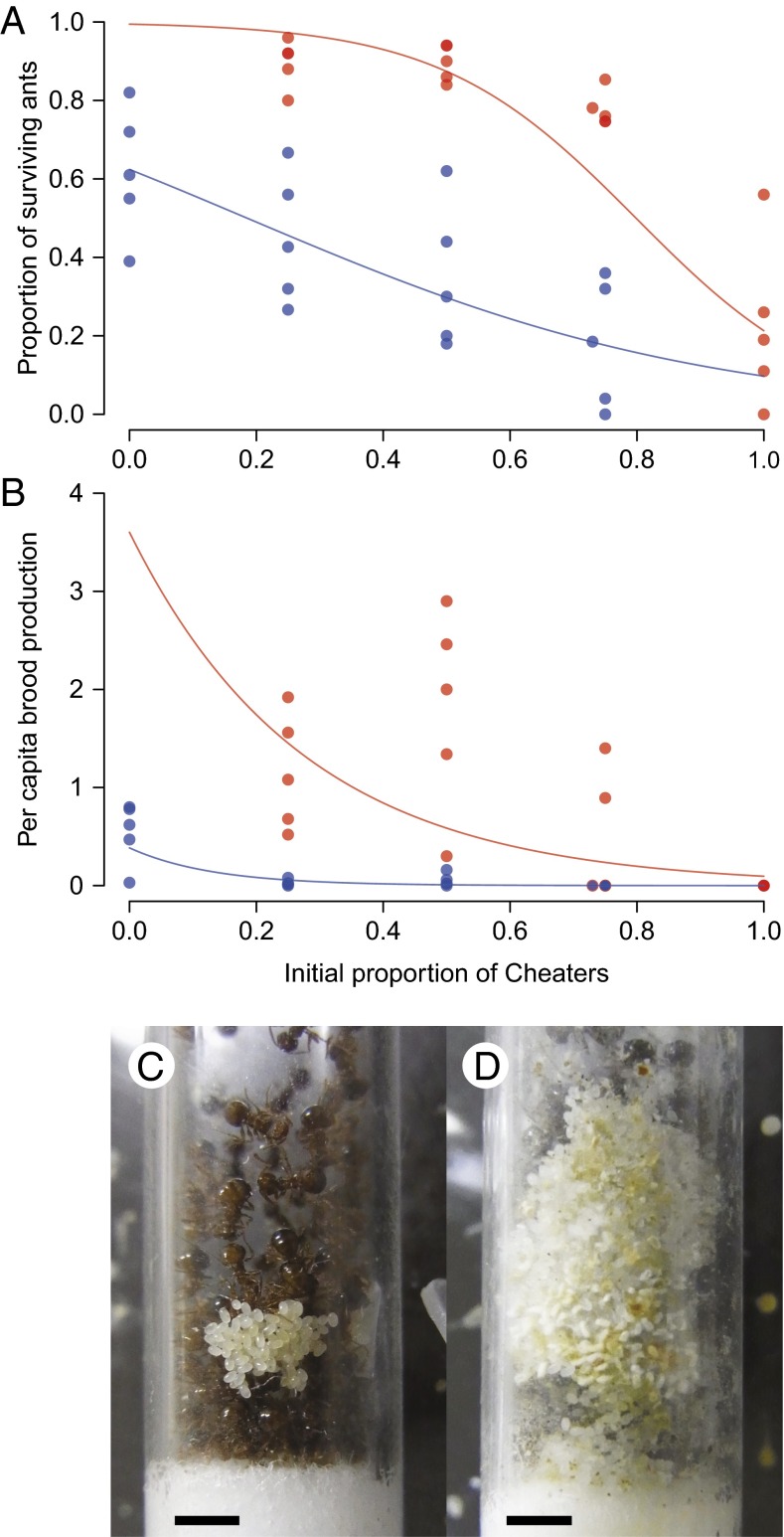
After 64 d of rearing, an increase in the initial proportion of cheaters significantly reduced the fitness components of individuals, in both survival [generalized linear mixed model (GLMM) with binomial errors and logit-link; workers: slope ± SEM = −2.734 ± 0.268; likelihood-ratio test (LRT), 1 df, χ2 = 114.4, P < 0.0001; cheaters: slope ± SEM = −6.483 ± 0.394; LRT, 1 df, χ2 = 427.7, P < 0.0001] (Fig. 1A) and brood production, based on the number of own eggs, larvae, and prepupae produced per ant, determined by microsatellite genotyping (GLMM with Poisson error and log-link; workers: slope ± SEM = −7.636 ± 0.637; LRT, 1 df, χ2 = 348.9, P < 0.0001; cheaters: slope ± SEM = −3.633 ± 0.156; LRT, 1 df, χ2 = 608.5, P < 0.0001) (Fig. 1B). Particularly, colonies with 100% cheaters failed to produce offspring (Fig. 1B). Another GLMM analysis comparing individual fitness between workers and cheaters (coded as 0 and 1, together with the initial cheater proportion included as explanatory variables) revealed that cheaters had significantly greater individual fitness than workers living with them, in both survival (GLMM with binomial errors and logit-link; slope ± SEM = 2.797 ± 0.161; LRT, 1 df, χ2 = 420.1, P < 0.0001) and brood production (GLMM with Poisson error and log-link; slope ± SEM = 3.733 ± 0.214; LRT, 1 df, χ2 = 955.0, P < 0.0001). These results conform to the framework of the public goods dilemma, which was further supported by a survival analysis using Cox’s proportional hazard model (SI Text and Fig. S1) and by observations of the colonies on days 16 (Fig. 1 C and D), 32, and 48 (SI Text and Fig. S2).
Fig. 1.
(A and B) Fitness components of cheaters (red) and workers (blue) after 64 d of rearing: (A) survival rate; (B) number of brood per capita. Each dot corresponds to an experimental colony. Regression curves were obtained from GLMMs to which data of workers and cheaters were fit independently. Note that colonies consisting of only cheaters failed to produce brood. (C and D) Snapshots of nests with (C) workers only (0% cheaters) and (D) cheaters only (100% cheaters) on day 16. In cheater-only colonies, more eggs were initially produced than in worker-only colonies, but they were neglected; some eggs even began to decay. The cheater-only colonies were also characterized by poor hygiene in the nests. (Scale bars, 2 mm.)
Colony-Level Task Allocation and Worker Behavior.
Among social insects, social regulation of the division of labor often achieves remarkable robustness or homeostasis against both external and internal perturbations (43–47), attributable to the behavioral flexibility of colony members that respond directly or indirectly to the perturbations. The underlying mechanism has been a key issue in the study of self-organization in biological systems (46, 47). In this study, we regarded the invasion of cheaters as another class of perturbation inherent in cooperative systems. Although it is well known that the reproductive division of labor is vulnerable to cheater invasion, less is known about other aspects of social insect division of labor in the face of cheaters, such as cooperative task allocation among workers.
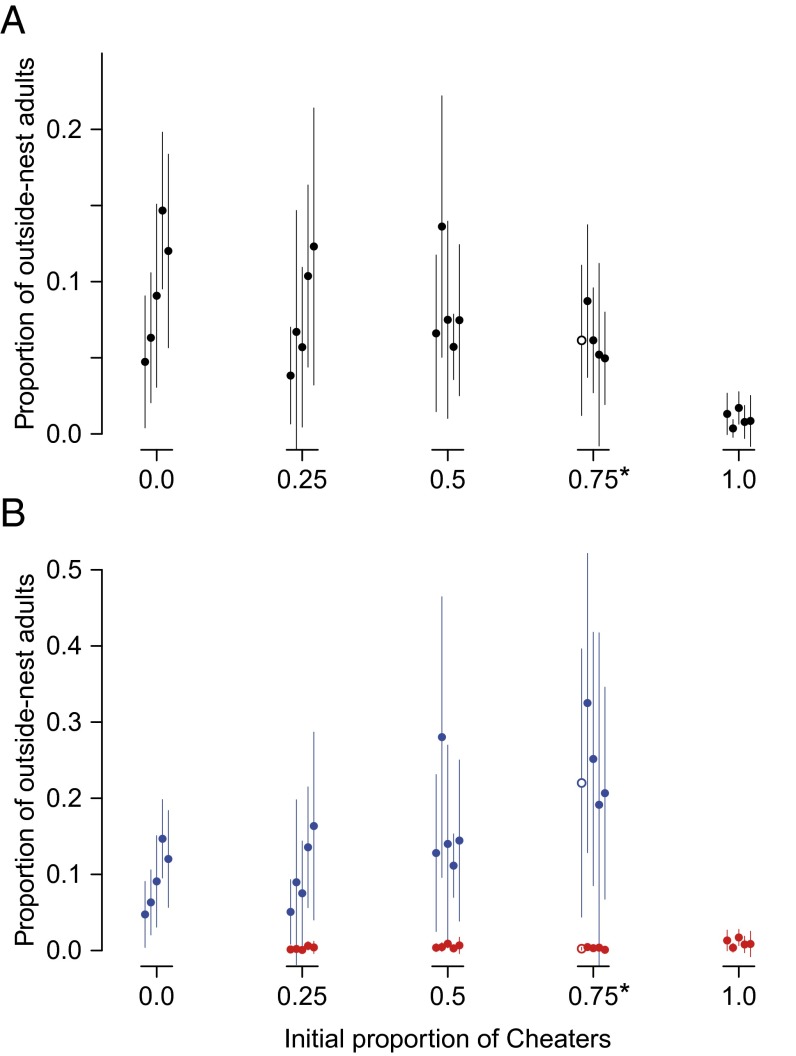
We focused on the robustness and flexibility of the allocation of tasks to activity outside the nest, because it is a typical cooperative behavior shown by postreproductive workers of ants. The proportions of individuals (workers + cheaters) found outside their nests, averaged over observation periods, was 3.8–14.7% in the presence of workers (0–75% cheaters) (Fig. 2A), but only 0.4–1.7% in the absence of workers (100% cheaters). When we introduced the presence/absence of workers (coded as 0/1) as an explanatory variable separate from the initial proportion of cheaters, the proportion of outside-nest individuals did not change significantly with the cheater proportion (GLMM with binomial errors and logit-link; LRT, 1 df, χ2 = 1.663, P = 0.1973), but the effect of worker absence was negative and significant (slope ± SEM = –1.799 ± 0.296; LRT, 1 df, χ2 = 23.07, P < 0.0001). These results indicate that the outside-nest activity of the colonies remained stable as long as enough workers were present to carry out all tasks. In addition, the proportion of outside-nest individuals increased significantly over time (effect of days from onset, slope ± SEM = 0.048 ± 0.001; LRT, 1 df, χ2 = 1,364, P < 0.0001).
Fig. 2.
Proportional allocation to outside-nest activity. Each dot corresponds to an experimental colony. To obtain representative data for the colonies, we calculated the weighted average and SD (error bars) over six observation periods (on days 10, 15, 20, 25, 30, and 35) for each colony, in which 10 replicates within a period were simply averaged. (A) Proportion of individuals (cheaters + workers) that were found outside the nest. Outside-nest activity remained stable as long as workers were present (0–75% cheaters). (B) Proportion of cheaters (red) and workers (blue) that were found outside the nest. Workers disproportionately increased their outside-nest activity as the cheater proportion increased. *Open circles indicate a colony with an initial cheater proportion of 0.73.
Next we analyzed who contributed to the outside-nest activity. In another GLMM, we included the individual’s affiliation (cheater/worker here coded as 0/1) as an explanatory variable. Workers had a significantly higher probability of engaging in outside-nest activity than cheaters (GLMM with binomial errors and logit-link; slope ± SEM = 4.197 ± 0.079; LRT, type II, 1 df, χ2 = 8,830, P < 0.0001) (Fig. 2B), and the outside-nest activity increased significantly over time (effect of days from onset, slope ± SEM = 0.052 ± 0.001; LRT, type II, 1 df, χ2 = 1,500, P < 0.0001). The analysis also revealed that the outside-nest activity of individuals increased significantly with the increase in the initial cheater proportion (slope ± SEM = 1.979 ± 0.271; LRT, type II, 1 df, χ2 = 29.47, P < 0.0001), and that workers contributed significantly more to the increase in the outside-nest activity than cheaters (interaction of cheater/worker × initial cheater proportion, slope ± SEM = 1.949 ± 0.393; LRT, type II, 1 df, χ2 = 24.54, P < 0.0001) (Fig. 2B).
Survival Cost of Workers Associated with Outside-Nest Activity.
Finally, we examined whether workers suffered from their forced switch to outside-nest activity. Because the outside-nest activity of workers was positively associated with initial cheater proportion and days from onset (Fig. 2B), we controlled for these effects in the proportion of outside-nest workers by using another GLMM (Table S1), and computed Pearson residuals (averaged over observations) that represented a pure effect of worker outside-nest activity of each colony during the observation periods.
The short-term survival of adult workers, as estimated from data during days 0–35, significantly decreased with their outside-nest activity (GLMM with binomial errors and logit-link; slope ± SEM = –4.700 ± 1.926; LRT, 1 df, χ2 = 5.660, P = 0.0174). However, the survival was not affected by the initial cheater proportion (LRT, 1 df, χ2 = 2.626, P = 0.1051). This result was in stark contrast with another analysis using data during days 36–64, when the worker survival rate did not show a significant association with outside-nest activity (GLMM with binomial errors and logit-link; LRT, 1 df, χ2 = 1.594, P = 0.2068), but significantly decreased with the initial cheater proportion (slope ± SEM = –2.668 ± 0.663; LRT, 1 df, χ2 = 10.95, P = 0.0009).
Discussion
Direct measurement of fitness using laboratory colonies enabled us to confirm that the interaction of cheaters and workers in P. punctatus meets the conditions of the public goods dilemma. We discuss the evolutionary implications arising from the dilemma, and illustrate the evolutionary convergence between microbial societies and P. punctatus societies that is found not only in general fitness consequences but also in behavioral mechanisms of cooperator–cheater interactions.
Coexistence of Cooperators and Cheaters.
In inclusive fitness theory, higher genetic relatedness between members within a group (denoted by r), as well as a greater benefit of cooperation (b) relative to its cost (−c), is the primary condition favoring cooperation in a group-structured population, summarized by the well-known Hamilton’s rule, rb − c > 0 (48). Studies of experimental evolution using microbial cooperative systems have demonstrated the importance of high relatedness in their evolution (22, 49). In P. punctatus, our previous population genetics study showed that the rate of worker migration among colonies in the study population was very low, at ∼10−5 per generation (40). The restriction of migration is likely explained by the operation of a nestmate discrimination system [although condition-dependent to some extent (51–54)]. In addition, genetic heterogeneity reduces colonies’ tendency to self-reassemble and possibly leads to reduced colony productivity and survival (55). These factors are summarized by high relatedness between nestmate workers. Adult individuals have a lifespan of approximately 1 y, whereas colonies can last far longer, are potentially immortal, and reproduce by colony fission (50). Once invading a colony, cheaters should increase in frequency within the colony over generations and eventually lead to collapse of the whole colony. If the relatedness structure were shared between workers and cheaters, cheaters would decrease in frequency by the microevolutionary process of kin selection (56) and would finally disappear in the field population.
Nevertheless, the actual presence of genetic cheaters with the apparent evolutionary dead-end strategy (57) in the field population of P. punctatus poses a question to their persistence. Our previous study indicated that cheaters had a much higher migration rate among colonies than cooperators, estimated at 10−2 per generation (40). Therefore, cheaters can avert the immediate extinction of their own lineage by acting like a “transmissible social cancer” (40, 58). The differential migration rates might be explained by the adaptation of the cheaters during the time they coexist with worker lineages [estimated at approximately 200–9,200 generations (40)]. The longer the coexistence time, the more coevolutionary options become available, allowing counter adaptation. Among the options is policing, by which selfish individuals are punished by their cooperative (or sometimes selfish) nestmates when their excessively high reproductive status is detected (59). It is worth noting that no evidence of policing (e.g., aggression toward adult cheaters) has been found so far in P. punctatus, although policing is widespread among social Hymenoptera (ants, bees, and wasps), even in some asexual species (60–62). In the long run, antagonistic coevolution between cheater and worker lineages, together with the virtual absence of genetic admixture between the two lineages (40), should make the intraspecific cooperator–cheater system resemble an interspecific host–social parasite relationship (63). More generally, evolutionary bifurcation of cheater lineages from the cooperator ancestor through antagonistic coevolution might provide another mechanism that protects cooperation and ensures the irreversibility of the major transitions in evolution (1, 4, 64).
Frequency Dependence of Fitness.
One of the convergent properties between microbial systems and the P. punctatus system is frequency-dependent selection; that is, the relative fitness of cheaters (and cooperators) is dependent on the frequency of cooperators (65). The frequency dependence stems from strong selection that is caused by genes with large fitness effects in the sufficiently structured population and results in population growth rates positively associated with cooperator frequencies (65). In both microbial and P. punctatus systems, a large difference in the degree of contribution to the public goods between cooperators and cheaters is the source of the strong selection.
Social Resilience and Behavioral Plasticity of Workers.
The average proportions of outside-nest adults (workers + cheaters) were not significantly affected by cheater proportions as long as enough workers were present (Fig. 2A). The observed range (3.8–14.7%) is close to previous field data (mean ± SD = 5.9% ± 2.7%) (56). A previous experimental study (excluding cheaters) showed that offspring production was maximized when 5–10% of the nestmates were postreproductive workers who worked outside the nest (66). These studies suggest the presence of regulatory mechanisms [called social resilience (43)] that maintain the optimum proportion of outside-nest individuals and still work against cheater invasion. The self-organized regulation in P. punctatus was attributable to flexible and compensatory switching from inside-nest to outside-nest tasks by workers in response to the increased cheater proportions (Fig. 2B). However, the increased outside-nest activity resulted in the reduced workforce inside the nest (as indicated by the hygiene status of the nest) (SI Text and Fig. S2) and imposed an additional survival cost on workers in the first half of the breeding period (days 0–35). The latter cost was likely because of the greater workload of outside-nest tasks or to the harsher environment than inside the nest (67, 68).
In social insects, dominance interactions of another ant species revealed a mechanistically similar cooperator-specific fitness cost (69), whereby a survival cost was initially imposed on subordinate workers because of an increased workload to match the enhanced reproductive activity of dominant sister workers. However, unlike the dominance interactions, workers and cheaters of P. punctatus are genetically distinct, and thus the costly behavioral flexibility of the workers offers no inclusive fitness return as long as they help unrelated nestmate cheaters. In social microbes, on the other hand, the quorum-sensing bacterium Pseudomonas aeruginosa shows a remarkable convergence with P. punctatus in the context of phenotypic plasticity of cooperators in response to cheaters (70). In P. aeruginosa, the costly contribution to the public goods (i.e., siderophore production) per cooperator cell increased with the increase in the proportion of unrelated cheater cells. In both fitness consequences and behavioral mechanisms, the striking degree of evolutionary convergence between microbial societies and P. punctatus societies illustrates that a wide range of scales of cooperative systems are governed by identical basic principles of social evolution.
Materials and Methods
Colonies.
Colonies of P. punctatus were collected from Kihoku, Mie Prefecture, central Japan (34.2°N, 136.3°E) (39, 40), on June 4 and 5, 2011, during the early to middle period of their reproductive season. Five colonies containing a substantial number of cheaters served as the source colonies for the experiment. Cheater adults are easily distinguishable from workers by their characteristically large body size and three distinct ocelli (workers have none) (42). To avoid potential confounding effects caused by a queen-like phenotype sporadically found in cooperative “noncheater” lineages (39–42), we used only individuals with the worker phenotype to represent “worker” lineages. Cheaters were first removed from the source colonies, and then the workers from a given colony were well mixed on a tray so as to avoid biased sampling of temporal castes. Brood were carefully removed from the initial experimental colonies. We established 25 experimental colonies each consisting initially of 100 individuals with 0%, 25%, 50%, 75%, or 100% cheaters (five colonies for each proportion), except for one colony, which contained 73% instead of 75% of cheaters because of a shortage of cheaters in the source colony. Each colony was maintained in a plastic case (105 mm × 105 mm × 55 mm) with poly(ethylene-cotetrafluoroethylene)-coated sides to stop the ants from climbing out and with a test tube (8-mm diameter and 90-mm length) that was covered by red plastic film and contained approximately 2 mL of distilled water that was kept separate from the rest of the test tube by a cotton plug. The ants nested inside the tube. Colonies were kept in the laboratory at 25 °C under a 16:8-h light:dark light cycle and were fed on 0.5 g of artificial diet (modified from ref. 71) every day (1800 hours to 2100 hours). Leftovers observed in all colonies indicated sufficient feed supply.
Observation of Task Allocation.
On days 10, 15, 20, 25, 30, and 35 from the onset, we recorded the total numbers of cheaters and workers outside the nests in each colony. Observations were conducted before feeding time, because feeding might induce recruitment of additional individuals that otherwise would not leave the nest (66). Each observation period consisted of a set of 10 30-s observations 12.5 min apart.
Measurement of Fitness Components.
Survival of the adult ants was monitored every day during feeding. Dead ants were phenotyped (cheater/worker) and removed from the cases. On days 16, 32, and 48, we checked inside the nests for the presence and developmental stage of brood (eggs and larvae, eggs only, or none) and for the hygiene condition. The results confirmed the progression of the public goods dilemma (SI Text and Figs. S1 and S2).
On day 64, all colonies were frozen, and the brood (eggs, larvae, and prepupae) was separated from the adults and prepared for genotyping. DNA was extracted individually on FTA cards (GE Healthcare), and genotyped at three polymorphic microsatellite loci (Pp2, ppmb114, and ppmb132; the resulting multilocus genotypes turned out to be diagnostic) as previously described (40). Of 1,059 individuals genotyped, five from four of the experimental colonies had no diagnostic allele combinations. The four colonies contained 25% or 50% cheaters, and >86.4% of the remaining brood were assigned as cheaters. The five individuals’ genotypes were consistent with those derived from cheaters through rare recombination events (heterozygotes to homozygotes) or with allele-specific DNA degradation during preservation. Therefore, we tentatively assigned them as cheaters.
Statistical Analyses.
In the GLMM analyses, the LRT was used to test for the statistical significance of the inclusion of each explanatory variable, with the maximum-likelihood estimation using the Laplace approximation. In GLMMs to which we fit datasets of workers and cheaters independently, the source colony ID was included as a random effect (random intercept). Otherwise the experimental colony ID (shared environment between workers and cheaters) nested within its source colony ID was included as a random intercept. In the model of per-capita brood production, the initial number of individuals was used as an offset term, which meant that the reproductive success was attributed to adults initially introduced into the experimental colonies. In the analysis of worker survival cost, we first computed the Pearson residual of the proportion of outside-nest workers at each observation using a GLMM (Table S1). Then the residuals were averaged over observations for each experimental colony (the weighted average was used, taking worker numbers into account), and served as a new explanatory variable that represented the workers’ pure outside-nest activity during the 10–35 d from the onset. All analyses were conducted using the “car,” “lme4,” “MASS,” and “survival” packages implemented in R v3.0.0 software (72).
Supplementary Material
Acknowledgments
We thank M. K. Hojo for field support; T. Abe, R. Kiyuna, F. Mitsube, M. Shimada, S. Sugimoto, and T. Tsuchimatsu for laboratory support; E. Hasegawa, H. Mori, T. Sasaki, and H. Shimoji for discussion; L. Keller for hosting S.D. at University of Lausanne; and S. A. West and an anonymous referee for comments on an earlier draft. S.D. was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (18-11584, 22-9877).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309010110/-/DCSupplemental.
References
- 1.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. New York: Oxford Univ Press; 1995. [Google Scholar]
- 2.Bourke AFG. Principles of Social Evolution. New York: Oxford Univ Press; 2011. [Google Scholar]
- 3.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211(4489):1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 4.Doebeli M, Hauert C. Models of cooperation based on the Prisoner’s Dilemma and the Snowdrift game. Ecol Lett. 2005;8(7):748–766. [Google Scholar]
- 5.Nowak MA. Evolutionary Dynamics. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]
- 6.Hauert C, Michor F, Nowak MA, Doebeli M. Synergy and discounting of cooperation in social dilemmas. J Theor Biol. 2006;239(2):195–202. doi: 10.1016/j.jtbi.2005.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher JA, Zwick M. The evolution of altruism: Game theory in multilevel selection and inclusive fitness. J Theor Biol. 2007;245(1):26–36. doi: 10.1016/j.jtbi.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Frank SA. A general model of the public goods dilemma. J Evol Biol. 2010;23(6):1245–1250. doi: 10.1111/j.1420-9101.2010.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milinski M. TIT FOR TAT in sticklebacks and the evolution of cooperation. Nature. 1987;325(6103):433–435. doi: 10.1038/325433a0. [DOI] [PubMed] [Google Scholar]
- 10.Dugatkin LA. Cooperation Among Animals. An Evolutionary Perspective. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 11.Noë R. Cooperation experiments: Coordination through communication versus acting apart together. Anim Behav. 2006;71(1):1–18. [Google Scholar]
- 12.Ledyard J. Public goods: A survey of experimental research. In: Kagel J, Roth A, editors. Handbook of Experimental Economics. Princeton: Princeton Univ Press; 1995. pp. 253–279. [Google Scholar]
- 13.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425(6960):785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 14.Gintis H, Bowles S, Boyd R, Fehr E. Moral Sentiments and Material Interests: The Foundations of Cooperation in Economic Life. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 15.Burton-Chellew MN, West SA. Prosocial preferences do not explain human cooperation in public-goods games. Proc Natl Acad Sci USA. 2013;110(1):216–221. doi: 10.1073/pnas.1210960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner PE, Chao L. Prisoner’s dilemma in an RNA virus. Nature. 1999;398(6726):441–443. doi: 10.1038/18913. [DOI] [PubMed] [Google Scholar]
- 17.Velicer GJ, Kroos L, Lenski RE. Developmental cheating in the social bacterium Myxococcus xanthus. Nature. 2000;404(6778):598–601. doi: 10.1038/35007066. [DOI] [PubMed] [Google Scholar]
- 18.Vulic M, Kolter R. Evolutionary cheating in Escherichia coli stationary phase cultures. Genetics. 2001;158(2):519–526. doi: 10.1093/genetics/158.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rainey PB, Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425(6953):72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 20.Greig D, Travisano M. The Prisoner’s Dilemma and polymorphism in yeast SUC genes. Proc Biol Sci. 2004;271(Suppl 3):S25–S26. doi: 10.1098/rsbl.2003.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert OM, Foster KR, Mehdiabadi NJ, Strassmann JE, Queller DC. High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proc Natl Acad Sci USA. 2007;104(21):8913–8917. doi: 10.1073/pnas.0702723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450(7168):411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 23.Raymond B, West SA, Griffin AS, Bonsall MB. The dynamics of cooperative bacterial virulence in the field. Science. 2012;337(6090):85–88. doi: 10.1126/science.1218196. [DOI] [PubMed] [Google Scholar]
- 24.Burt A, Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Cambridge, MA: Belknap Press of Harvard Univ Press; 2006. [Google Scholar]
- 25.Barron AB, Oldroyd BP, Ratnieks FLW. Worker reproduction in honey-bees (Apis) and the anarchic syndrome: A review. Behav Ecol Sociobiol. 2001;50(3):199–208. [Google Scholar]
- 26.Jordan LA, Allsopp MH, Oldroyd BP, Wossler TC, Beekman M. Cheating honeybee workers produce royal offspring. Proc Biol Sci. 2008;275(1632):345–351. doi: 10.1098/rspb.2007.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilley CA, Oldroyd BP. Unequal subfamily proportions among honey bee queen and worker brood. Anim Behav. 1997;54(6):1483–1490. doi: 10.1006/anbe.1997.0546. [DOI] [PubMed] [Google Scholar]
- 28.Hughes WOH, Boomsma JJ. Genetic royal cheats in leaf-cutting ant societies. Proc Natl Acad Sci USA. 2008;105(13):5150–5153. doi: 10.1073/pnas.0710262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chéron B, Monnin T, Fédérici P, Doums C. Variation in patriline reproductive success during queen production in orphaned colonies of the thelytokous ant Cataglyphis cursor. Mol Ecol. 2011;20(9):2011–2022. doi: 10.1111/j.1365-294X.2011.05075.x. [DOI] [PubMed] [Google Scholar]
- 30.Osborne KE, Oldroyd BP. Possible causes of reproductive dominance during emergency queen rearing by honeybees. Anim Behav. 1999;58(2):267–272. doi: 10.1006/anbe.1999.1139. [DOI] [PubMed] [Google Scholar]
- 31.Moritz RFA, et al. Rare royal families in honeybees, Apis mellifera. Naturwissenschaften. 2005;92(10):488–491. doi: 10.1007/s00114-005-0025-6. [DOI] [PubMed] [Google Scholar]
- 32.Holzer B, Kümmerli R, Keller L, Chapuisat M. Sham nepotism as a result of intrinsic differences in brood viability in ants. Proc Biol Sci. 2006;273(1597):2049–2052. doi: 10.1098/rspb.2006.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwander T, Keller L. Genetic compatibility affects queen and worker caste determination. Science. 2008;322(5901):552. doi: 10.1126/science.1162590. [DOI] [PubMed] [Google Scholar]
- 34.Wiernasz DC, Cole BJ. Patriline shifting leads to apparent genetic caste determination in harvester ants. Proc Natl Acad Sci USA. 2010;107(29):12958–12962. doi: 10.1073/pnas.1003299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobata S, Tsuji K. Intragenomic conflict over queen determination favours genomic imprinting in eusocial Hymenoptera. Proc Biol Sci. 2012;279(1738):2553–2560. doi: 10.1098/rspb.2011.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itow T, et al. The reproductive cycle of the queenless ant Pristomyrmex pungens. Insectes Soc. 1984;31(1):87–102. [Google Scholar]
- 37.Tsuji K. Obligate parthenogenesis and reproductive division of labor in the Japanese queenless ant Pristomyrmex pungens: Comparison of intranidal and extranidal workers. Behav Ecol Sociobiol. 1988;23(4):247–255. [Google Scholar]
- 38.Tsuji K. Reproductive division of labour related to age in the Japanese queenless ant Pristomyrmex pungens. Anim Behav. 1990;39(5):843–849. [Google Scholar]
- 39.Dobata S, et al. Cheater genotypes in the parthenogenetic ant Pristomyrmex punctatus. Proc Biol Sci. 2009;276(1656):567–574. doi: 10.1098/rspb.2008.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobata S, et al. Persistence of the single lineage of transmissible ‘social cancer’ in an asexual ant. Mol Ecol. 2011;20(3):441–455. doi: 10.1111/j.1365-294X.2010.04954.x. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki T, Tsuji K. Behavioral property of unusual large workers in the ant, Pristomyrmex pungens. J Ethol. 2003;21(2):145–151. [Google Scholar]
- 42.Tsuji K, Dobata S. Social cancer and the biology of the clonal ant Pristomyrmex punctatus (Hymenoptera: Formicidae) Myrmecol News. 2011;15:91–99. [Google Scholar]
- 43.Sendova-Franks AB, Franks NR. Social resilience in individual worker ants and its role in division of labour. Proc Biol Sci. 1994;256(1347):305–309. [Google Scholar]
- 44.Jones JC, Myerscough MR, Graham S, Oldroyd BP. Honey bee nest thermoregulation: Diversity promotes stability. Science. 2004;305(5682):402–404. doi: 10.1126/science.1096340. [DOI] [PubMed] [Google Scholar]
- 45.Robinson EJH, Feinerman O, Franks NR. Flexible task allocation and the organization of work in ants. Proc Biol Sci. 2009;276(1677):4373–4380. doi: 10.1098/rspb.2009.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detrain C, Deneubourg J-L, Pasteels JM. Information Processing in Social Insects. Basel, Switzerland: Birkhauser; 1999. [Google Scholar]
- 47.Camazine S, et al. Self-Organization in Biological Systems. Princeton, NJ: Princeton Univ Press; 2001. [Google Scholar]
- 48.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7(1):17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 49.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430(7003):1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 50.Tsuji K. Nest relocations in the Japanese queenless ant Pristomyrmex pungens Mayr (Hymenoptera: Formicidae) Insectes Soc. 1988;35(4):321–340. [Google Scholar]
- 51.Tsuji K. Inter-colonial incompatibility and aggressive interactions in Pristomyrmex pungens (Hymenoptera: Formicidae) J Ethol. 1988;6(2):77–81. [Google Scholar]
- 52.Tsuji K. Kin recognition in Pristomyrmex pungens (Hymenoptera: Formicidae): Asymmetrical change in acceptance and rejection due to odour transfer. Anim Behav. 1990;40(2):306–312. [Google Scholar]
- 53.Sanada-Morimura S, et al. Encounter-induced hostility to neighbours in the ant Pristomyrmex pungens. Behav Ecol. 2003;14(5):713–718. [Google Scholar]
- 54.Satow S, Satoh T, Hirota T. Colony fusion in a parthenogenetic ant, Pristomyrmex punctatus. J Insect Sci. 2013;13:38. doi: 10.1673/031.013.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishide Y, Satoh T, Hiraoka T, Obara Y, Iwabuchi K. Clonal structure affects the assembling behavior in the Japanese queenless ant Pristomyrmex punctatus. Naturwissenschaften. 2007;94(10):865–869. doi: 10.1007/s00114-007-0267-6. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji K. Reproductive conflicts and levels of selection in the parthenogenetic ant, Pristomyrmex pungens: Contextual analysis and partitioning of covariance. Am Nat. 1995;146:586–607. [Google Scholar]
- 57.Pruitt JN. A real-time eco-evolutionary dead-end strategy is mediated by the traits of lineage progenitors and interactions with colony invaders. Ecol Lett. 2013;16(7):879–886. doi: 10.1111/ele.12123. [DOI] [PubMed] [Google Scholar]
- 58.Dobata S, Tsuji K. A cheater lineage in a social insect: Implications for the evolution of cooperation in the wild. Commun Integr Biol. 2009;2(2):67–70. doi: 10.4161/cib.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ratnieks FLW, Foster KR, Wenseleers T. Conflict resolution in insect societies. Annu Rev Entomol. 2006;51:581–608. doi: 10.1146/annurev.ento.51.110104.151003. [DOI] [PubMed] [Google Scholar]
- 60.Pirk CWW, Neumann P, Ratnieks FLW. Cape honeybees, Apis mellifera capensis, police worker-laid eggs despite the absence of relatedness benefits. Behav Ecol. 2002;14(3):347–352. [Google Scholar]
- 61.Hartmann A, Wantia J, Torres JA, Heinze J. Worker policing without genetic conflicts in a clonal ant. Proc Natl Acad Sci USA. 2003;100(22):12836–12840. doi: 10.1073/pnas.2132993100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teseo S, Kronauer DJC, Jaisson P, Châline N. Enforcement of reproductive synchrony via policing in a clonal ant. Curr Biol. 2013;23(4):328–332. doi: 10.1016/j.cub.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Brandt M, Foitzik S, Fischer-Blass B, Heinze J. The coevolutionary dynamics of obligate ant social parasite systems—Between prudence and antagonism. Biol Rev Camb Philos Soc. 2005;80(2):251–267. doi: 10.1017/s1464793104006669. [DOI] [PubMed] [Google Scholar]
- 64.Doebeli M, Hauert C, Killingback T. The evolutionary origin of cooperators and defectors. Science. 2004;306(5697):859–862. doi: 10.1126/science.1101456. [DOI] [PubMed] [Google Scholar]
- 65.Ross-Gillespie A, Gardner A, West SA, Griffin AS. Frequency dependence and cooperation: Theory and a test with bacteria. Am Nat. 2007;170(3):331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 66.Tsuji K. Inter-colonial selection for the maintenance of cooperative breeding in the ant Pristomyrmex pungens: A laboratory experiment. Behav Ecol Sociobiol. 1994;35(2):109–113. [Google Scholar]
- 67.Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton, NJ: Princeton Univ Press; 1978. [PubMed] [Google Scholar]
- 68.Bourke AFG, Franks NR. Social Evolution in Ants. Princeton, NJ: Princeton Univ Press; 1995. [Google Scholar]
- 69.Tsuji K, Kikuta N, Kikuchi T. Determination of the cost of worker reproduction via diminished life span in the ant Diacamma sp. Evolution. 2012;66(5):1322–1331. doi: 10.1111/j.1558-5646.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- 70.Kümmerli R, Jiricny N, Clarke LS, West SA, Griffin AS. Phenotypic plasticity of a cooperative behaviour in bacteria. J Evol Biol. 2009;22(3):589–598. doi: 10.1111/j.1420-9101.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 71.Bhatkar A, Whitcomb WH. Artificial diet for rearing various species of ants. Fla Entomol. 1970;53(4):229–232. [Google Scholar]
- 72.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.