Figure 6.
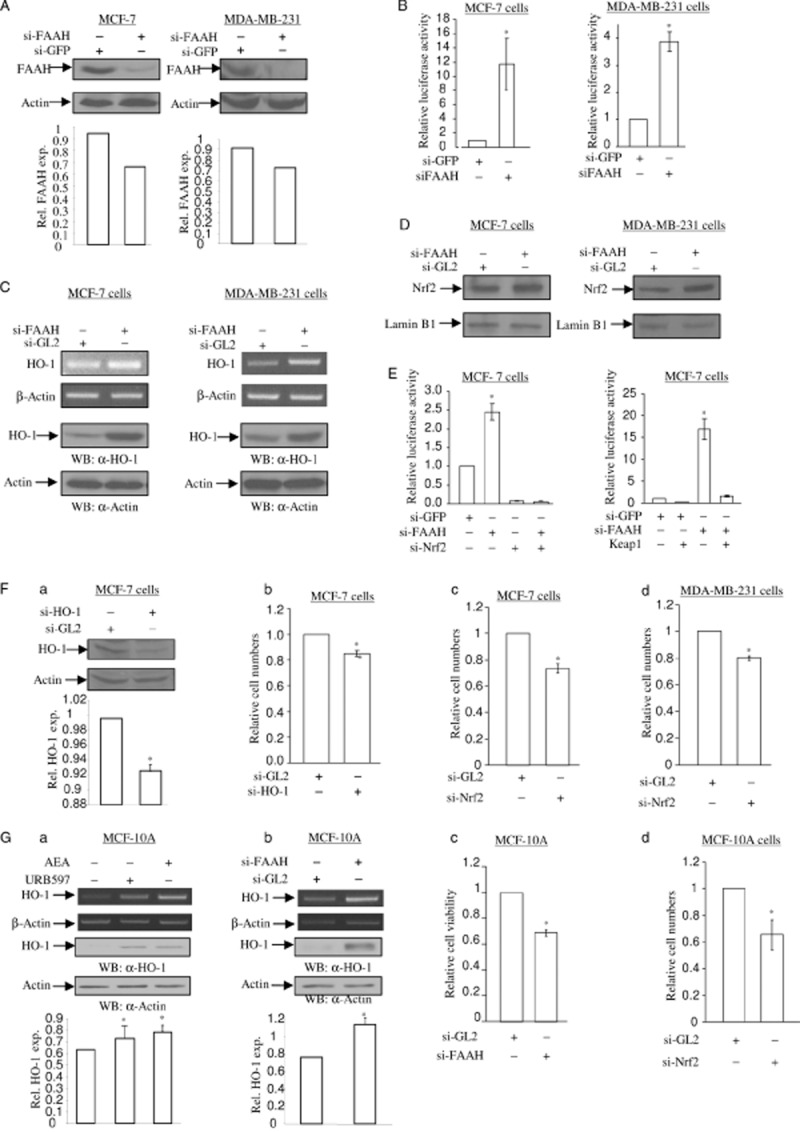
Depletion of FAAH increases HO-1 mRNA and protein expression. (A) Depletion of endogenous FAAH protein after si-FAAH RNA treatment. MCF-7 cells (left) and MDA-MB-231 cells (right) were treated with 5 nM of si-GL2 RNA (control) and si-FAAH RNA. After 72 h, total cell lysates were subjected to SDS-PAGE and immunoblotting to detect the levels of FAAH protein. The same membrane was blotted with anti-actin antibody to monitor equal loading of proteins. The levels of FAAH protein were normalized to actin levels and the relative expression was represented graphically. All the figures are representative of three independent experiments. (B) Depletion of FAAH activates nqo1-ARE-Luc reporter. MCF-7 cells and MDA-MB-231 cells were treated with 5 nM of si-GFP RNA (control) and si-FAAH RNA. After 16 h, cells were introduced with 0.5 μg of nqo1-ARE-Luc reporter and 0.15 μg of pCMV-β-galactosidase plasmids. After 24 h, luciferase activity assay and data analysis were performed as in Figure 1C. The data represent the mean ± SD of at least three independent experiments performed in triplicate. (C) Depletion of FAAH induces HO-1 mRNA and protein expression. MCF-7 cells (left) and MDA-MB-231 cells (right) were treated with 5 nM of si-GL2 RNA (control) and si-FAAH RNA. After 48 h, semi-quantitative RT-PCR for HO-1 and β-actin mRNA (top two panels) was performed as in Figure 1A. In a parallel experiment, total protein was subjected to SDS-PAGE and immunoblotting in order to detect the levels of HO-1 protein. The same membrane was blotted with anti-actin antibody to monitor equal loading of proteins. (D) Depletion of FAAH induces Nrf2 nuclear translocation. MCF-7 cells (left) and MDA-MB-231 cells (right) were treated with 5 nM of si-GL2 RNA (control) and si-FAAH RNA respectively. After 48 h, the nuclear proteins were separated and 10 μg of protein extract was subjected to SDS-PAGE and immunoblotting in order to detect the levels of Nrf2 protein. The same membrane was blotted with anti-lamin B1 antibody to monitor equal loading of nuclear proteins. All figures are representative of three independent experiments. (E) Nrf2 is involved in si-FAAH treatment-activated nqo1-ARE-Luc reporter activity. On the left side, depletion of Nrf2 abolishes si-FAAH RNA treatment-activated nqo1-ARE-Luc reporter activity. MCF-7 cells were treated with 5 nM of si-GFP RNA, 2.5 nM of si-FAAH RNA and 2.5 nM of si-Nrf2 RNA, in combination as indicated. After 16 h, cells were introduced with 0.5 μg of nqo1-ARE-Luc reporter and 0.15 μg of pCMV-β-galactosidase plasmids. After 24 h, luciferase activity assay and data analysis were performed as Figure 1C. The data represent the mean ± SD of at least three independent experiments performed in triplicate. On the right side, overexpression of Keap1 blocks si-FAAH RNA treatment-activated nqo1-ARE-Luc reporter activity. MCF-7 cells were co-expressed with 0.5 μg of nqo1-ARE-Luc reporter and 0.15 μg of pCMV-β-galactosidase plasmids, with or without Keap1 plasmid. After 24 h, luciferase activity assay and data analysis were performed as in Figure 1C. The data represent the mean ± SD of at least three independent experiments performed in triplicate. (F) Depletion of HO-1 causes a decrease in cell numbers in MCF-7 and MDA-MB-231 cells. (a) Knockdown of endogenous HO-1 by si-HO-1 RNA treatment. MCF-7 cells and MDA-MB-231 cells were treated with 5 nM of si-GL2 RNA (control) and si-HO-1 RNA. After 72 h, total cell lysates were subjected to determine the levels of HO-1 protein. The same membrane was blotted with anti-actin antibody to monitor equal loading of proteins. The levels of HO-1 protein were normalized to the actin levels and the relative expression was represented graphically. All figures are representative of three independent experiments. (b) Depletion of HO-1 reduces cell numbers. MCF-7 cells treated with 5 nM of si-GL2 RNA (control) and si-HO-1 RNA were stained with crystal violet at 72 h. The cells were extracted in 1% SDS solution and the absorbance was determined at 570 nm. Changes in cell numbers were obtained upon comparison of the OD570 between si-GL2 RNA-treated cells and si-HO-1 RNA-treated cells. The data represent the mean ± SD of at least three independent experiments performed in triplicate. Depletion of Nrf2 decreases cell numbers in MCF-7 (c) and MDA-MB-231 (d) cells. MCF-7 cells and MDA-MB-231 cells treated with 5 nM of si-GL2 RNA (control) and si-Nrf2 RNA were stained with crystal violet at 72 h. The cells number assay was performed as Figure 6F-b. The data represent the mean ± SD of at least three independent experiments performed in triplicate. (G) AEA signalling in MCF-10A immortalized breast epithelial cells. (a, b) URB597, AEA and si-FAAH RNA increase the levels of HO-1 mRNA and protein in MCF-10A cells. In Figure 6G, MCF-10A cells were treated with 5 μM of URB597 and 2.5 μM of AEA for 6 and 16 h for semi-quantitative RT-PCR of HO-1 mRNA and immunoblotting of HO-1 protein respectively. β-Actin primers were used as a control for semi-quantitative RT-PCR and anti-actin antibody was applied to monitor equal loading for immunoblotting. MCF-10A cells treated with 5 nM of si-FAAH RNA and si-GL2 RNA (control) for 3 days were subjected to semi-quantitative RT-PCR for HO-1 mRNA and immunoblotting of HO-1 protein respectively. β-Actin primers were used as a control for semi-quantitative RT-PCR and anti-actin antibody was applied to monitor equal loading for immunoblotting. The levels of HO-1 protein were normalized to actin levels and the relative expression was represented graphically. All the figures are representative of three independent experiments. Depletion of HO-1 and Nrf2 decrease cell numbers in MCF-10A cells. MCF-10A cells were treated with 5 nM of si-FAAH RNA (c), si-Nrf2 RNA (d) and si-GL2 RNA (control) as indicated. At 72 h, the cells were stained with crystal violet as Figure 6F-b. The data represent the mean ± SD of at least three independent experiments performed in triplicate.
