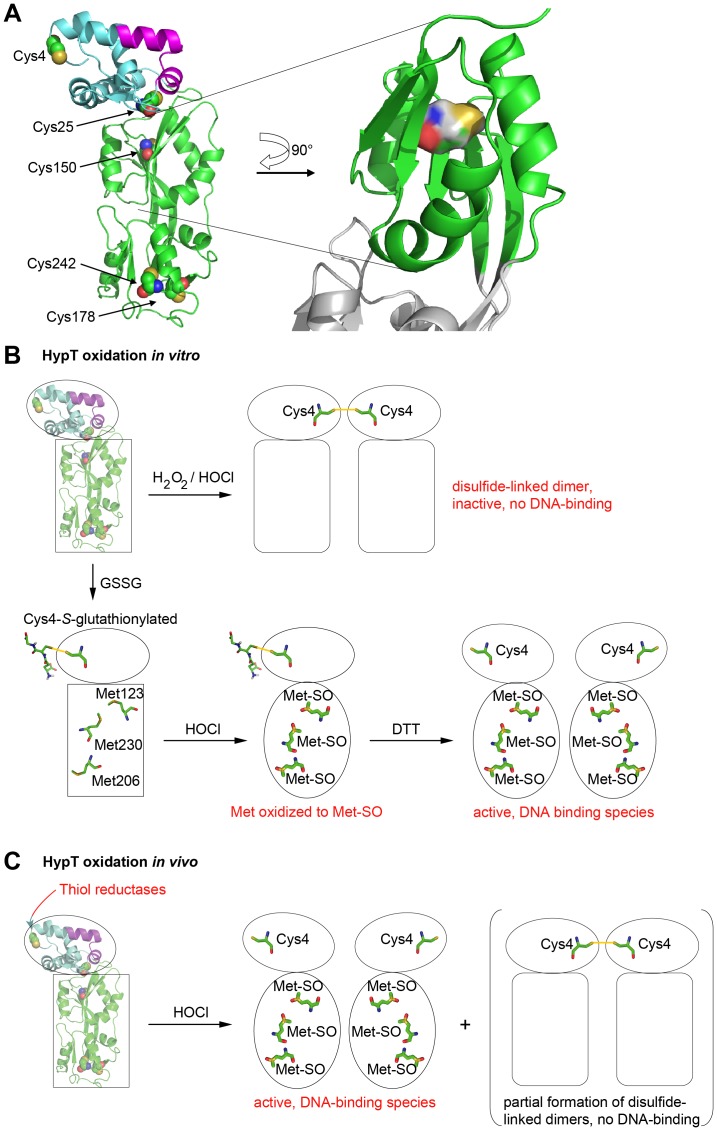
Figure 6. Structural model of HypT and working model.
A. Secondary structure prediction of HypT using the prediction software Phyre 2. The cysteines are indicated and the location of Cys150 emphasized. B. Model of HypT function upon oxidative stress in vitro. Oxidation of purified HypT leads to disulfide bond formation between Cys4 of one HypT molecule with Cys4 of another HypT molecule and concomitant conformational changes in the N-terminal DNA-binding domain and/or C-terminal domain leading to inhibited DNA binding. Upon reversible protection of cysteine residues by S-glutathionylation, specific activation of HypT by HOCl and generation of the active DNA-binding species occurs. Also here, conformational changes occur in HypT, which potentially positions the DNA-binding domain for efficient DNA-binding. Please note that the exact conformational changes are unknown. C. Model of HypT function upon oxidative stress in vivo. HOCl treatment of cells causes methionine oxidation and conformational changes leading to activation of HypT. Cellular thiol reductases maintain HypT cysteines mostly in the reduced state. As a side reaction, partial Cys4-Cys4 disulfide bond formation may occur.