Figure 1.
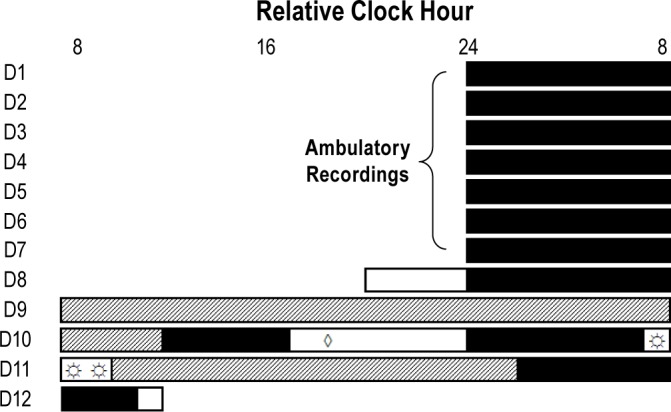
12 daylong phase-shifting protocol. The first 7 days (D1-7) of study were ambulatory recording of subjects maintaining consistent sleep-wakefulness schedules. This was followed by 5 days living in the laboratory (D8-12). Example times for a subject with a habitual bedtime at midnight. Black bars represent scheduled sleep opportunities, open bars represent scheduled wakefulness not part of constant routines, and the hatched bars represent constant routines. The protocol consisted of 2 constant routines which were used to estimate circadian phase. Subjects received melatonin or rice powder-filled placebo (◊) 5.75 h prior to habitual bedtime. Subjects were awakened from sleep early so that exposure to bright or dim light (☼) could begin 1 h prior to habitual wake time.
