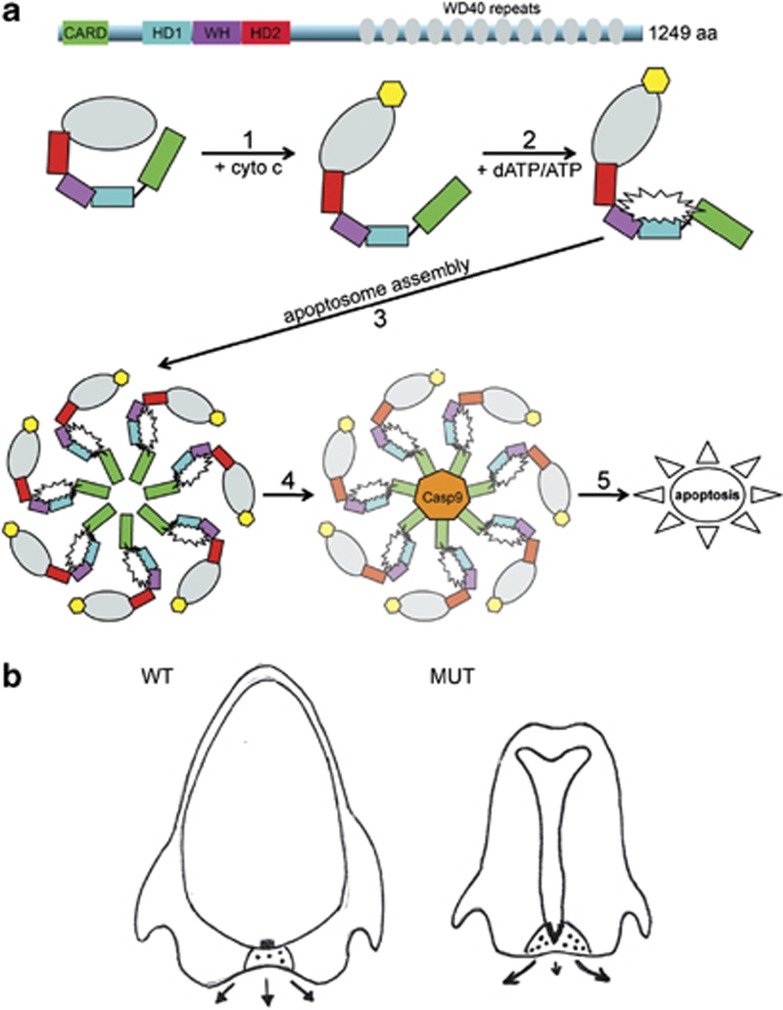
Figure 6.
Model of Apaf1yautja action. (a) In the cytoplasm, Apaf1 exists as an autoinhibited monomer, wherein the WD40 repeats (gray) interact with the N-terminal domains. Step 1: cytochrome c (yellow), released from the mitochondria, binds to the WD40 repeats and a conformational change occurs in Apaf1. Step 2: the binding of dATP/ATP (white starburst) to the interface between the nucleotide-binding domain (black line), HD1 (blue) and the WH domain (purple), allows further conformational changes that promote apoptosome assembly. Step 3: seven molecules of Apaf1 associate with each other to form the apoptosome, with protein–protein interactions being critical for stability and function. Step 4: procaspase 9 (orange) associates with the apoptosome and becomes active, causing the downstream activation of effector caspase 3. Step 5: cleavage of targets associated with programmed cell death and apoptosis of the cell. The L375P mutation in Apaf1yautja, in the WH domain, could alter the ability of steps 2, 3 and/or 4 to occur, or decrease their efficiency. (b) Expanded Shh (black boxes) in the prechordal plate of Apaf1yautja embryos (MUT) acts on the mesenchyme of the neighboring FNP (black dots). This causes increased proliferation and directed growth away from the midline (arrows, with length of arrow indicating amount of outgrowth)