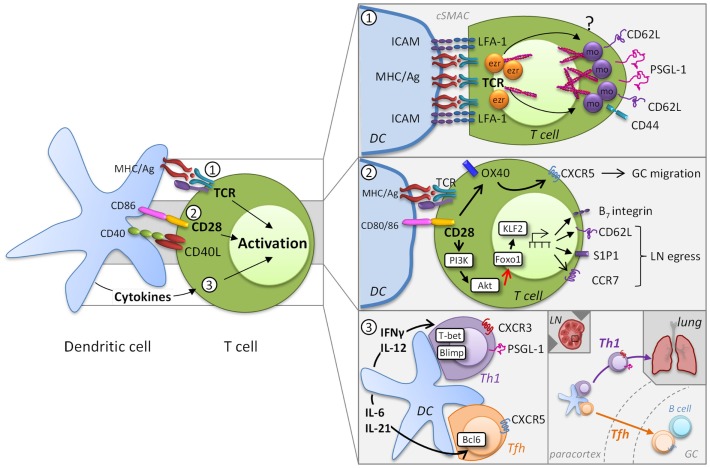
Figure 1.
Induction of migratory heterogeneity during priming. MR phenotype is impacted by TCR engagement, the level of co-stimulation, and the cytokine milieu (left). MR play a direct role in the formation of the immunological synapse, but TCR signaling subsequently impacts MR expression [panel 1, adapted from Ref. (8)]. Cytoskeletal rearrangements that involve the actin-binding ezrin (ezr), radixin, and moesin (mo) proteins are necessary for TCR signaling complex polarization. The integrin LFA-1 forms a ring surrounding the cSMAC that supports prolonged T cell-DC engagement, while other MR become excluded from the cSMAC. This process is possibly due to differential polarization of ezrin and moesin. Co-stimulatory signaling through molecules also contributes to the migratory heterogeneity of T cells (panel 2). For example, CD28 controls migration through upregulation of OX40, which is instrumental for CXCR5 expression and T cell localization to germinal centers (GC). In addition CD28/TCR signaling activates the PI3K/AKT pathway, which inhibits Foxo1 leading to decreased KLF2 expression. Differential co-stimulation can impact the levels of CD62L, CCR7, and S1P1 and thereby regulate the egress of T cells into the circulation. Cytokines released by DC promote specific transcriptional profiles that introduce further MR heterogeneity (panel 3). DC-derived IL-12 induces expression of the transcription factor T-bet and determines a CD4+ Th1 or CD8+ effector transcriptional program that results in part in the expression of CXCR3 and PSGL-1, which contribute to homing to peripheral sites. Alternatively, induction of the Tfh-associated transcription factor Bcl6 by IL-6 and IL-21 results in the downregulation of PSGL-1 and increased expression of CXCR5, which allows these cells to migrate from the T cell zones in the paracortex into GC.