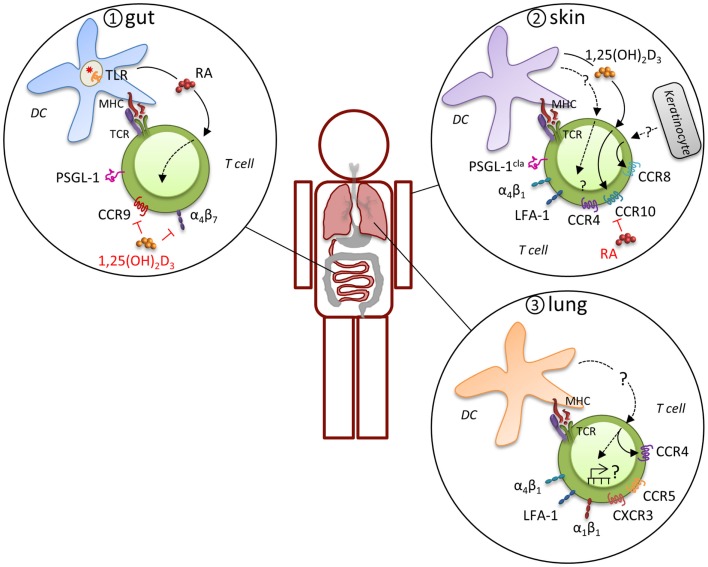
Figure 2.
Induction of tissue-specific MR profile. Expression of distinct MR combinations results in tissue-specific migration. T cells that are primed in gut-associated lymphoid tissues preferentially express functional PSGL-1, α4β7, and CCR9, which support migration through the post capillary venules of the small intestine to enter the lamina propria and intraepithelial compartment (panel 1). CD103-expressing DC impart the intestinal homing signature on T cells during priming by expression of retinoic acid (RA) in response to microbiota-driven toll-like receptor (TLR) signaling. A different program of MR usage is induced during priming of naïve T cells in skin-draining LN (panel 2). T cells in the skin express the cutaneous lymphocyte antigen (CLA), an inducible carbohydrate modification of PSGL-1, CCR4, CCR8, CCR10, α4β1, and LFA-1, which mitigates their migration into the skin. Analogous to intestinal imprinting, CCR10, but not CCR4, expression is regulated by skin-draining DCs that synthesize the vitamin D3 metabolite, 1,25(OH)2D3. 1,25(OH)2D3 suppresses α4β7 and CCR9 expression and RA inhibits CCR4 and CCR10 expression. In addition, CCR8 expression is imprinted by epidermal keratinocytes, although the skin-specific factors that induce CCR8 remain unknown. Evidence for imprinting of T cells in the lung is limited, but recent evidence suggests that lung DC-activated T cell migrate more efficiently into the lung, which was attributed to CCR4, although other MR are likely to contribute (panel 3). Many lymphocytes in the lung express high levels of α4β1, α1β1, and LFA-1 and CD8+ T cells primed in the mediastinal LN are enriched for CCR5 and CXCR3 expression, suggesting a pulmonary profile driven by distinct molecular mechanisms.