Abstract
This study examines the role of aqueous secondary organic aerosol formation in the North American Sonoran Desert as a result of intense solar radiation, enhanced moisture, and biogenic volatile organic compounds (BVOCs). The ratio of water-soluble organic carbon (WSOC) to organic carbon (OC) nearly doubles during the monsoon season relative to other seasons of the year. When normalized by mixing height, the WSOC enhancement during monsoon months relative to preceding dry months (May–June) exceeds that of sulfate by nearly a factor of 10. WSOC:OC and WSOC are most strongly correlated with moisture parameters, temperature, and concentrations of O3 and BVOCs. No positive relationship was identified between WSOC or WSOC:OC and anthropogenic tracers such as CO over a full year. This study points at the need for further work to understand the effect of BVOCs and moisture in altering aerosol properties in understudied desert regions.
1. Introduction
The chemical complexity and uncertainties in production mechanisms of atmospheric aerosol species pose a challenge for assessments of aerosol effects on public health and global climate. Little is known about the nature of organic aerosols in arid areas such as the Sonoran Desert of southwestern North America (Southwest). In recent decades, the Southwest has experienced significant urbanization, land use change, and is potentially moving toward a more arid regime with higher temperatures and lower soil moisture [Seager et al., 2007; Cayan et al., 2010]. Such changes are expected to alter aerosol physicochemical properties through changes in pollutant emissions and temperature-dependent processes affecting formation mechanisms and sinks.
A topic of large uncertainty is aqueous-phase (wet aerosol particles and cloud droplets) production of secondary organic aerosol (SOA) [Ervens et al., 2011]. Multiphase processes include the partitioning of soluble organic vapors into the aqueous phase and the possibility for subsequent chemistry to produce lower volatility species that remain in the aerosol phase upon subsequent evaporation of water. The Sonoran Desert is a suitable natural laboratory to examine the role of aqueous-phase production of SOA due to the North American Monsoon season (July–September), characterized by influx of moisture, increased vegetation growth, and higher levels of biogenic volatile organic compounds (BVOCs). The intense insolation in desert ecosystems enhances photochemistry and biological emissions [Guenther et al., 1993; Diem, 2000; Diem and Comrie, 2000], such as from the native Larrea tridentata (creosote bush) [Geron et al., 2006; Jardine et al., 2010].
The goal of this study is to examine potential influences of moisture and BVOCs on aerosol in the Sonoran Desert. The conclusions of this analysis rely on water-soluble organic carbon (WSOC) and its ratio to other organic parameters as proxies for SOA formation, as has been done elsewhere [e.g., Miyazaki et al., 2006; Sullivan et al., 2006; Kondo et al., 2007; Weber et al., 2007; Hennigan et al., 2008a, 2008b; Hennigan et al., 2009; Peltier et al., 2008; Duong et al., 2011; Hersey et al., 2011].
2. Experimental Methods
2.1. Aerosol, Gas, and Meteorological Data
Daily PM2.5 aerosol measurements were conducted from June 2011 to February 2013 in a rooftop laboratory (30 m above ground level (agl), 720 m above sea level (asl)) of the Physics and Atmospheric Sciences Building on the University of Arizona campus in Tucson, Arizona (32.23°N, 110.95°W, metropolitan population ~ 1 million; U.S. Census Bureau, 2009). Water-soluble organic carbon (WSOC) was measured at 6 min time resolution with a Particle-Into-Liquid Sampler (Brechtel Manufacturing Inc.) coupled to a total organic carbon analyzer (Sievers, Model 800) [Sullivan et al., 2006]. Measurement uncertainty is ~10% with a minimum detection limit of 0.1 µg m−3. More specific instrument operational details are provided elsewhere [Wonaschuetz et al., 2011]. A semicontinuous OC/EC analyzer (Sunset Laboratory Inc., Oregon) measured hourly organic carbon (OC) and elemental carbon (EC) mass concentrations [Birch, 1998] (Minimum detection limits of OC and EC: 1 µg m−3 and 0.2 µg m−3, respectively; measurement uncertainty ~20%). Cloud condensation nuclei (CCN) concentrations at 0.2% supersaturation were measured with a CCN counter (CCN-100, DMT Inc.) [Roberts and Nenes, 2005]. Submicrometer aerosol number concentrations (CN) were obtained with a scanning mobility particle sizer (SMPS 3936L, TSI Inc.). The CCN:CN ratio is referred to here as the “activation ratio” and used as a proxy for aerosol hygroscopicity.
Hourly measurements of ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), and carbon monoxide (CO) were obtained for the year 2011 from a nearby (~9 km) surface pollutant monitoring site operated by the Pima County Department of Environmental Quality (Children's Park Station). Hourly meteorological data (relative humidity (RH), temperature, atmospheric pressure, and water vapor mixing ratio (WVMR)) were collected adjacent to the roof-top laboratory. Sulfate concentrations were obtained from two EPA Interagency Monitoring of Protected Visual Environments (IMPROVE) (http://views.cira.colostate.edu/web/) [Malm et al., 1994] monitoring stations within 25 km of the rooftop laboratory: Saguaro West (years 2002–2009; 32.25°N, −111.22°W; 718 m asl) and Saguaro National Monument (years 2000–2009; 32.12°N, −110.74°W; 933 m asl). Mixing heights were calculated from a Weather and Research Forecasting (WRF) model run using the Yonsei University boundary layer scheme on a 36 km grid [Hu et al., 2010].
2.2. Quantification of Biogenic Emissions
Three proxies for BVOC emissions are used: (i) the Normalized Difference Vegetation Index (NDVI) product from the Moderate Resolution Imaging Spectroradiometer for the period between June 2011 and December 2012 and (ii–iii) modeled surface concentrations of isoprene and monoterpenes. The BVOCs were obtained at 6 h time resolution and 1.9° × 2.5° spatial resolution for the period between June 2011 and August 2012 from MOZART-4/GEOS5 simulations (http://www.acd.ucar.edu/wrf-chem/mozart.shtml) [Emmons et al., 2010] driven by meteorological input from the NASA GMAO GEOS-5 model. The BVOC surface fluxes were calculated using the Model of Emission of Gases and Aerosols from Nature [Guenther et al., 2006]. While the spatial resolution of these products exceeds that of the Tucson area, the relative changes in BVOC emissions on a regional scale agree with those in the smaller spatial domain of the surface measurements [Diem, 2000; Diem and Comrie, 2001a, 2001b], especially over the monthly and seasonal time scales examined here.
3. Study Site Description and Meteorology
Tucson is surrounded by mountain ranges and characterized by diverse types of vegetation. Desert areas are covered with shrubs (e.g., creosote bush) and small trees. Urban areas have considerable amounts of leaf biomass owing to native species (e.g., palo verde and creosote bush) and exotic species such as eucalyptus [Diem, 2000]. The mountain ranges are forested with pines, junipers, oaks, and firs. In the arid climate with hot summers and mild winters (Figure 1), precipitation falls in two modes: December– March and July–September (monsoon). Mixing height, typically less than 400 m agl in the winter and near 1.5 km agl in July (Figure 1), is a significant factor in governing PM2.5 and O3 levels [Wise and Comrie, 2005a, 2005b]. Five seasons are defined in this study: Fall (October–November), Winter (December–February), Spring (March–April), Dry Summer (May–June), and Monsoon Summer (July–September). Higher WVMR define the Monsoon Summer (Figure 1). The study region can be impacted by wildfires, which are most frequent during May and June before the onset of monsoon precipitation [Sorooshian et al., 2011] but did not influence the measurements during the Monsoon Summer in this study. Primary biological aerosol particle (PBAP) emissions, which can be an important source of WSOC, are concentrated in sizes above those examined here (Dp>2.5 µm) [e.g., Bauer et al., 2002]. Fungal spores account for 2 ± 1% of contemporary carbon in PM2.5 in nearby Phoenix during the summer [Holden et al., 2011], and fine-mode PBAP concentrations are predicted to rarely exceed 0.3 µg m−3 [Heald and Spracklen, 2009].
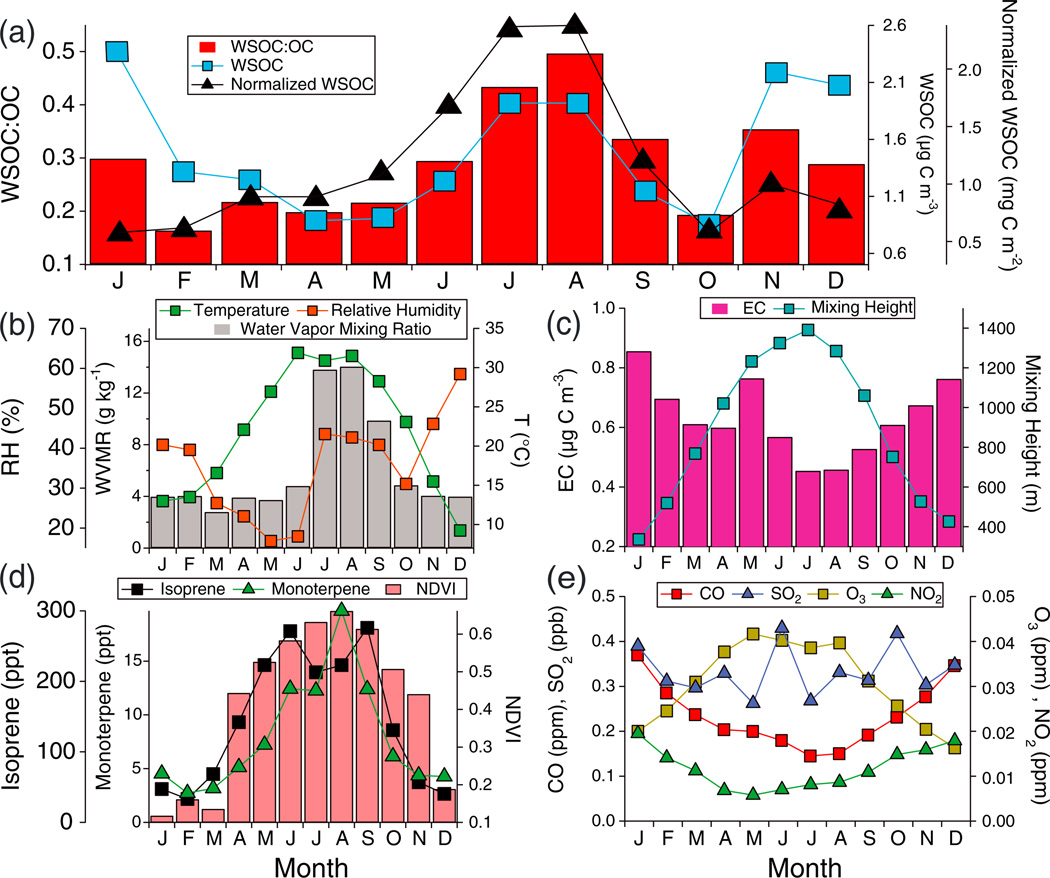
Figure 1.
Annual cycle of monthly averages of (a) WSOC, WSOC multiplied by mixing height (normalized WSOC), and the WSOC:OC ratio, (b) water vapor mixing ratio, RH, and temperature, (c) EC and mixing height, (d) BVOC emission proxies (NDVI, monoterpene, and isoprene), and (e) selected gas concentrations (O3, SO2, NO2, and CO).
4. Cumulative Results
The highest seasonal average concentrations of OC, EC, and WSOC are observed during winter (Table 1). This can be explained by shallower mixing heights and combustion processes for heating [Diem and Comrie, 2001b]. EC is directly emitted and less sensitive to seasonal variability in temperature, O3, and other factors influencing SOA production. Similar to EC, anthropogenic gas-phase species (CO and NO2) also follow the annual cycle driven by mixing height (Figure 1). The seasonal trends of OC and WSOC are different from EC and also each other, reflecting varying dependency on seasonally driven emissions and meteorology.
Table 1.
Seasonal Averages of OC, EC, WSOC, and Their Ratios (Average ± Standard Deviation)a
| OC (µgm−3) | EC (µgm−3) | WSOC (µgm−3) | WSOC:OC | |
|---|---|---|---|---|
| Fall | 4.94 ± 2.07 | 0.64 ± 0.43 | 1.39 ± 0.79 | 0.26 ± 0.12 |
| Winter | 7.03 ± 3.72 | 0.79 ± 0.60 | 1.90 ± 1.01 | 0.26 ± 0.11 |
| Spring | 4.29 ± 1.65 | 0.62 ± 0.35 | 0.94 ± 0.42 | 0.20 ± 0.06 |
| Dry Summer | 4.52 ± 2.15 | 0.60 ± 0.34 | 1.08 ± 0.73 | 0.26 ± 0.12 |
| Monsoon Summer | 4.24 ± 1.59 | 0.50 ± 0.31 | 1.64 ± 0.73 | 0.43 ± 0.16 |
Fall (October–November), Winter (December–February), Spring (March–April), Dry Summer (May–June), Monsoon Summer (July–September).
Recent work has suggested that WSOC can serve as a proxy for SOA in the absence of biomass burning and that its ratio relative to OC can provide insight into the degree of oxygenation of the organic fraction of the aerosol [Miyazaki et al., 2006; Kondo et al., 2007; Weber et al., 2007; Duong et al., 2011]. WSOC:OC and WSOC:EC exhibit a maximum during the Monsoon Summer (Table 1). WSOC:OC is generally lower than observed in Sweden, K-Puszta (Hungary), Italy, Jungfraujoch (Switzerland), St. Louis (United States), the southeastern United States, and various sites in the French Alps [Jaffrezo et al., 2005, and references therein; Sullivan and Weber, 2006; Zhang et al., 2012], but are comparable to observations in Tokyo (~0.20–0.35) [Miyazaki et al., 2006].
To investigate why WSOC shows contrasting behavior relative to total OC, several factors thought to govern its production are examined (Figure 1). WVMR and proxies for BVOC emissions closely follow the annual profile of WSOC:OC; RH also is enhanced during the Monsoon Summer relative to preceding months, but unlike WVMR, it is highest in the winter due to its sensitivity to temperature. A correlation analysis of factors potentially influencing absolute concentrations of WSOC and the WSOC:OC ratio (WVMR, RH, T, O3, NO2, CO, SO2, isoprene, monoterpenes) shows that the dominant factor is WVMR (r = 0.52 and r = 0.64, respectively; n = 489) (Table S1, Supplementary). The WSOC:OC ratio exhibits statistically significant correlations with O3 (r = 0.36), an indicator of photochemical processes and thus of photochemical production of WSOC from VOC precursors, and with surface concentrations of isoprene (r = 0.22) and monoterpenes (r = 0.36), but inverse correlations with NO2 (r =−0.27) and CO (r =−0.29).
WSOC formation via aqueous-phase processing depends not just on moisture levels but also on the affinity of particles to take up water vapor. The CCN activation ratio during the Monsoon Summer was 0.13 ± 0.06, higher than in all other seasons (averages ranged between 0.08 and 0.11), indicating that aerosols during the Monsoon Summer likely have the most liquid water associated with them due to a combination of more moisture and enhanced aerosol hygroscopicity.
The magnitude of WSOC enhancement as a result of seasonally dependent factors is quantified during the Monsoon Summer relative to the Dry Summer. The comparison of these two seasons leverages differences in WVMR (11.3 g kg−1 versus 4.2 g kg−1 for Monsoon Summer and Dry Summer, respectively), RH (42 ±1% versus 17 ± 1%), and CCN activity (0.13 ± 0.06 versus 0.11 ± 0.07) at comparable ambient temperatures (30.1°C versus 29.4°C) and O3 (0.036 ppm versus 0.040 ppm); similar values of temperature suggest that differences in the effects of volatilization from the aerosol phase are likely insignificant. When accounting for mixing height differences between the two seasons (i.e., multiplication of mass concentration by mixing height), the absolute enhancement in WSOC during the Monsoon Summer is 0.59 mg C m−2, a 42% increase relative to Dry Summer concentrations. The slope of the linear regression between WSOC and WVMR is 0.08 (mg C m−2)/(g kg−1). Sulfate, which is produced via aqueous-phase processing, exhibits a Monsoon Summer enhancement of 0.05 mg m−2 (3.2% increase) and 0.02 mg m−2 (1.4% increase) for SaguaroWest and Saguaro National Monument, respectively. The increase in WSOC exceeds that of sulfate by nearly a factor of 10 in the Monsoon Summer relative to the Dry Summer, suggestive of the seasonal influence of factors other than aerosol water content, most likely BVOC emissions. While modeled surface isoprene concentrations decrease from the Dry to Monsoon Summer (247.2 ppt versus 237.3 ppt, respectively), those of monoterpenes increase (9.8 ppt versus 14.8 ppt) and NDVI increases from 0.55 to 0.64. These results warrant a more detailed characterization of BVOC emissions in the study region using in situ measurements.
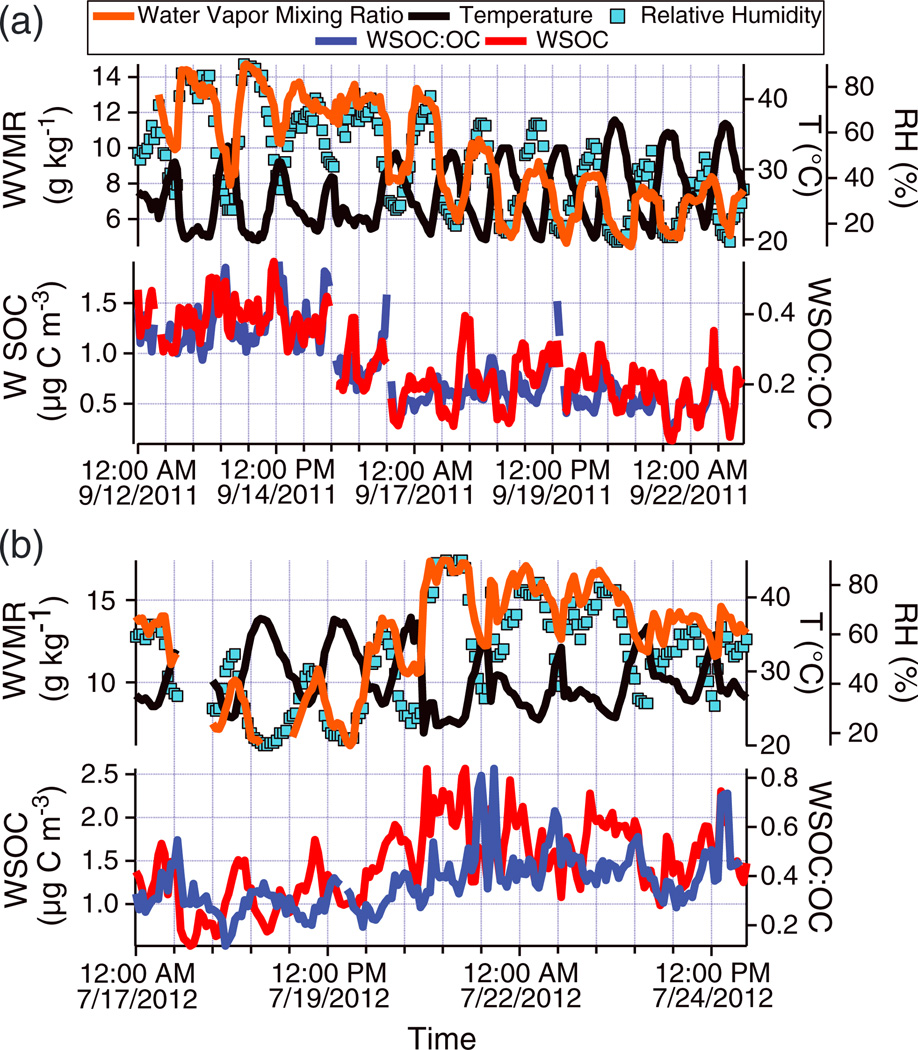
5. Case Studies in Monsoon Season
Two case studies of a clear shift between lower and higher RH and WVMR are presented in Figure 2 and show a trend of higher WSOC:OC values in the moister periods. The WSOC:OC ratio was higher by a factor of 1.95 and 1.43 in the moister periods of Case Studies 1 and 2, respectively (Table S2 in the supporting information). Furthermore, absolute WSOC concentrations were higher by a factor of 1.87 and 1.22 for the moist periods of Case Studies 1 and 2. The greater enhancement in Case Study 1 is consistent with the sharper change in the WVMR and RH from the dryer to the moister period (factor of 1.58 and 1.72, respectively) as compared to Case Study 2 (factor of 1.38 and 1.41). A correlation analysis for the two separate case studies indicates that the strongest relationship between any parameter and either WSOC or WSOC:OC is WVMR (r = 0.61 and 0.88; n = 37 and 23 for Case Studies 1 and 2, respectively) (Tables S3 and S4 in the supporting information). Similarly, high correlations between WSOC and water vapor (average r = 0.92 for 14 separate events) have been observed in the southeastern United States [Hennigan et al., 2008a]. Other factors potentially influencing WSOC:OC and WSOC (i.e., temperature, O3, anthropogenic, and biogenic VOCs) did not exhibit significant changes between the dryer and moister periods (Table S2 and Figure S1 in the supporting information).
Figure 2.
Time series of two case studies during the Monsoon Summer with a shift (a) from high to low moisture (12–22 Sep 2011)), and (b) from low to high moisture (17–24 Jul 2012)).
6. Conclusions
This study reports OC, EC, and WSOC concentrations in concert with other parameters potentially influencing aqueous SOA formation in the North American Sonoran Desert. The sharp influx of monsoon moisture and BVOC emissions between July and September coincide with major enhancements in WSOC and the WSOC:OC ratio. The increase in WSOC during the Monsoon Summer relative to the Dry Summer exceeds that of sulfate by nearly a factor of ten when accounting for mixing height changes, most likely due to seasonally dependent BVOC emissions. No positive relationship was identified between either WSOC or WSOC:OC with anthropogenic tracers such as CO over a full year. These results are especially of significance as recent model results show that aqueous SOA formation is geographically concentrated in the eastern United States and likely unimportant in other areas such as the Southwest [Carlton and Turpin, 2013]; more effort is warranted to bring models and measurements of aqueous SOA in agreement in a variety of regions including understudied deserts.
Supplementary Material
Acknowledgments
Authors J. Youn and Z. Wang contributed equally to this work. This research was supported in part by grant 2 P42 ES04940–11 from the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program, NIH and the University of Arizona TRIF Water Sustainability Program. Some of the analyses and visualizations used in this study were produced with the Giovanni online data system, developed and maintained by the NASA GES DISC. We acknowledge the sponsors of the IMPROVE network and the Pima County Department of Environmental Quality.
The Editor thanks two anonymous reviewers for their assistance in evaluating this paper.
Footnotes
Additional supporting information may be found in the online version of this article.
References
- Bauer H, et al. Determination of the carbon content of airborne fungal spores. Anal. Chem. 2002;74:91–95. doi: 10.1021/ac010331+. [DOI] [PubMed] [Google Scholar]
- Birch ME. Analysis of carbonaceous aerosols: Interlaboratory comparison. Analyst. 1998;123(5):851–857. doi: 10.1039/a800028j. [DOI] [PubMed] [Google Scholar]
- Carlton AG, Turpin BJ. Particle partitioning potential of organic compounds is highest in the Eastern US and driven by anthropogenic water. Atmos. Chem. Phys. Discuss. 2013;13:12,743–12,770. [Google Scholar]
- Cayan DR, Das T, Pierce DW, Barnett TP, Tyree M, Gershunov A. Future dryness in the southwest US and the hydrology of the early 21st century drought. Proc. Natl. Acad. Sci. U.S.A. 2010;107(50):21,271–21,276. doi: 10.1073/pnas.0912391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem JE. Comparisons of weekday-weekend ozone: Importance of biogenic volatile organic compound emissions in the semi-arid southwest USA. Atmos. Environ. 2000;34(20):3445–3451. [Google Scholar]
- Diem JE, Comrie AC. Integrating remote sensing and local vegetation information for a high-resolution biogenic emissions inventory—Application to an urbanized, semiarid region. J. Air Waste Manage. 2000;50(11):1968–1979. doi: 10.1080/10473289.2000.10464223. [DOI] [PubMed] [Google Scholar]
- Diem JE, Comrie AC. Air quality, climate, and policy: A case study of ozone pollution in Tucson, Arizona. Prof. Geogr. 2001a;53(4):469–491. [Google Scholar]
- Diem JE, Comrie AC. Allocating anthropogenic pollutant emissions over space: Application to ozone pollution management. J. Environ. Manage. 2001b;63(4):425–447. doi: 10.1006/jema.2001.0492. [DOI] [PubMed] [Google Scholar]
- Duong HT, et al. Water-soluble organic aerosol in the Los Angeles Basin and outflow regions: Airborne and ground measurements during the 2010 CalNex field campaign. J. Geophys. Res. 2011;116:D00V04. [Google Scholar]
- Emmons LK, et al. Description and evaluation of the model for ozone and related chemical tracers, version 4 (MOZART-4) Geosci. Model Dev. 2010;3(1):43–67. [Google Scholar]
- Ervens B, Turpin BJ, Weber RJ. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos. Chem. Phys. 2011;11(21):11,069–11,102. [Google Scholar]
- Geron C, et al. Biogenic volatile organic compound emissions from desert vegetation of the southwestern US. Atmos. Environ. 2006;40(9):1645–1660. [Google Scholar]
- Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability—Model evaluations and sensitivity analyses. J. Geophys. Res. 1993;98(D7):12,609–12,617. [Google Scholar]
- Guenther A, Karl T, Harley P, Wiedinmeyer C, Palmer PI, Geron C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmos. Chem. Phys. 2006;6:3181–3210. [Google Scholar]
- Heald CL, Spracklen DV. Atmospheric budget of primary biological aerosol particles from fungal spores. Geophys. Res. Lett. 2009;36:L09806. [Google Scholar]
- Hennigan CJ, Bergin MH, Weber RJ. Correlations between water-soluble organic aerosol and water vapor: A synergistic effect from biogenic emissions? Environ. Sci. Technol. 2008a;42(24):9079–9085. doi: 10.1021/es802189y. [DOI] [PubMed] [Google Scholar]
- Hennigan CJ, Bergin MH, Dibb JE, Weber RJ. Enhanced secondary organic aerosol formation due to water uptake by fine particles. Geophys. Res. Lett. 2008b;35:L18801. [Google Scholar]
- Hennigan CJ, Bergin MH, Russell AG, Nenes A, Weber RJ. Gas/particle partitioning of water-soluble organic aerosol in Atlanta. Atmos. Chem. Phys. 2009;9(11):3613–3628. [Google Scholar]
- Hersey SP, et al. The Pasadena aerosol characterization observatory (PACO): Chemical and physical analysis of the western Los Angeles Basin aerosol. Atmos. Chem. Phys. 2011;11:7417–7443. [Google Scholar]
- Holden AS, et al. Determining contributions of biomass burning and other sources to fine particle contemporary carbon in the western United States. Atmos. Environ. 2011;45:1986–1993. [Google Scholar]
- Hu XM, Nielsen-Gammon JW, Zhang FQ. Evaluation of three planetary boundary layer schemes in the WRF model. J. Appl. Meteorol. Climatol. 2010;49:1831–1844. [Google Scholar]
- Jaffrezo JL, Aymoz G, Delaval C, Cozic J. Seasonal variations of the water soluble organic carbon mass fraction of aerosol in two valleys of the French Alps. Atmos. Chem. Phys. 2005;5:2809–2821. [Google Scholar]
- Jardine K, Abrell L, Kurc SA, Huxman T, Ortega J, Guenther A. Volatile organic compound emissions from Larrea tridentata (creosote bush) Atmos. Chem. Phys. 2010;10(24):12,191–12,206. [Google Scholar]
- Kondo Y, et al. Oxygenated and water-soluble organic aerosols in Tokyo. J. Geophys. Res. 2007;112:D01203. [Google Scholar]
- Malm WC, Sisler JF, Huffman D, Eldred RA, Cahill TA. Spatial and seasonal trends in particle concentration and optical extinction in the United States. Geophys. Res. 1994;99(D1):1347–1370. [Google Scholar]
- Miyazaki Y, et al. Time-resolved measurements of water-soluble organic carbon in Tokyo. J. Geophys. Res. 2006;111:D23206. [Google Scholar]
- Peltier RE, et al. Investigating the sources and atmospheric processing of fine particles from Asia and the Northwestern United States measured during INTEX B. Atmos. Chem. Phys. 2008;8(6):1835–1853. [Google Scholar]
- Roberts GC, Nenes A. A continuous-flow streamwise thermal-gradient CCN chamber for atmospheric measurements. Aerosol Sci. Technol. 2005;39:206–221. [Google Scholar]
- Seager R, et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science. 2007;316(5828):1181–1184. doi: 10.1126/science.1139601. [DOI] [PubMed] [Google Scholar]
- Sorooshian A, et al. An aerosol climatology for a rapidly growing arid region (southern Arizona): Major aerosol species and remotely sensed aerosol properties. J. Geophys. Res. 2011;116:D19205. doi: 10.1029/2011JD016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AP, Weber RJ. Chemical characterization of the ambient organic aerosol soluble in water: 1. Isolation of hydrophobic and hydrophilic fractions with a XAD-8 resin. J. Geophys. Res. 2006;111:D05314. [Google Scholar]
- Sullivan AP, et al. Airborne measurements of carbonaceous aerosol soluble in water over northeastern United States: Method development and an investigation into water-soluble organic carbon sources. J. Geophys. Res. 2006;111:D23S46. [Google Scholar]
- Weber RJ, et al. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J. Geophys. Res. 2007;112:D13302. [Google Scholar]
- Wise EK, Comrie AC. Meteorologically adjusted urban air quality trends in the Southwestern United States. Atmos. Environ. 2005a;39(16):2969–2980. [Google Scholar]
- Wise EK, Comrie AC. Extending the Kolmogorov-Zurbenko filter: Application to ozone, particulate matter, and meteorological trends. J. Air Waste Manage. 2005b;55(8):1208–1216. doi: 10.1080/10473289.2005.10464718. [DOI] [PubMed] [Google Scholar]
- Wonaschuetz A, et al. Impact of a large wildfire on water-soluble organic aerosol in a major urban area: The 2009 Station Fire in Los Angeles County. Atmos. Chem. Phys. 2011;11(16):8257–8270. [Google Scholar]
- Zhang X, et al. Spatial and seasonal variations of fine particle watersoluble organic carbon (WSOC) over the southeastern United States: Implications for secondary organic aerosol formation. Atmos. Chem. Phys. 2012;12(14):6593–6607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.