Table 2.
Amination of chloropyrazine and 2-chloropyrimidine.[a]
| Entry | Amine | Product (yield [%]) for the reaction with | |
|---|---|---|---|
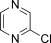 |
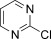 |
||
| 1 | 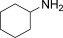 |
3 (28; MW 60[c]) | 11 (77) |
| 2 | 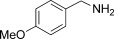 |
4 (47) | 12 (85) |
| 3 | 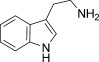 |
5 (58) | 13 (85) |
| 4 | 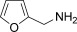 |
6 (33; MW 52[c]) | 14 (81) |
| 5 |  |
– | 15 (78) |
| 6 | 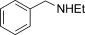 |
– | 16 (80) [95][b] |
| 7 | 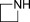 |
7 (48) | 17 (52) |
| 8 | 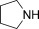 |
8 (76) | 18 (76) |
| 9 | 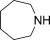 |
9 (52) | 19 (69) |
| 10 |  |
1 (70) [68, 86][b] | 2 (84) |
| 11 |  |
10 (81[d]) [93][b] | 20 (93) |
| 12 | 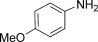 |
no reaction | 21 (86) [75, 83][b] |
| 14 | 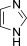 |
no reaction | 22 (62) |
| 15 | 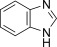 |
no reaction | 23 (83) |
All reactions were performed with heteroaryl halide (1 equiv.), amine (1 equiv.) and KF (2 equiv.) in water at 100 °C for 17 h.
Yields in square brackets refer to the palladium-catalysed variants shown in Scheme 1.
MW refers to the yield obtained if the reaction was performed in a microwave reactor (300 W) at 175 °C for 60 min with KF (1 equiv.).
Yield reported as an NMR yield calculated from the internal standard using 1,4-dioxane.
