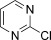
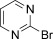
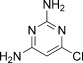
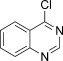
Table 3.
Amination of halopyrimidines and 4-chloroquinazoline.[a]
| Entry | Amine | Product (yield [%]) for the reaction with | |||
|---|---|---|---|---|---|
 |
 |
 |
 |
||
| 1 | 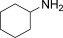 |
11 (77) | 11 (72) | no reaction | 27 (78) |
| 2 | 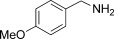 |
12 (85) | 12 (89) | no reaction | 28 (71) |
| 3 | 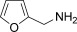 |
14 (81) | 14 (68) | no reaction | 29 (71) |
| 4 | 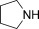 |
18 (76) | 18 (59) | 24 (45) | 30 (97) |
| 5 |  |
2 (84) | 2 (85) | 25 (49) | 31 (80) |
| 6 |  |
20 (93) | 20 (91) | 26 (80) | 32 (82) |
| 7 |  |
21 (86) | 21 (65) | no reaction | 33 (86) |
All reactions were performed with aryl halide (1 equiv.), amine (1 equiv.) and KF (2 equiv.) in water at 100 °C for 17 h.
