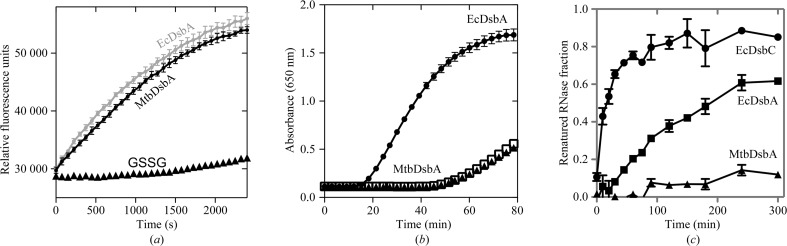
Figure 3.
Disulfide oxidoreductase activities. (a) Disulfide oxidase activity. Representative fluorescence curves of peptide cysteine oxidation by MtbDsbA and EcDsbA in the presence of glutathione as the electron donor. Enzyme-catalyzed peptide oxidation is significantly faster than the glutathione-mediated reaction. Peptide oxidation in the buffer control or by the catalytically inactive MtbDsbA (Cys89Ala) or EcDsbA (Cys33Ala) was insignificant over the duration of the assay (not shown for clarity). (b) Insulin disulfide-reduction assay. The precipitation of insulin by MtbDsbA or EcDsbA or DTT (trace overlaps that of EcDsbA) was monitored as described in §2. (c) Scrambled RNase disulfide isomerization assay. Disulfide isomerization activity of MtbDsbA, EcDsbA and EcDsbC was monitored using scrambled RNase as the substrate.