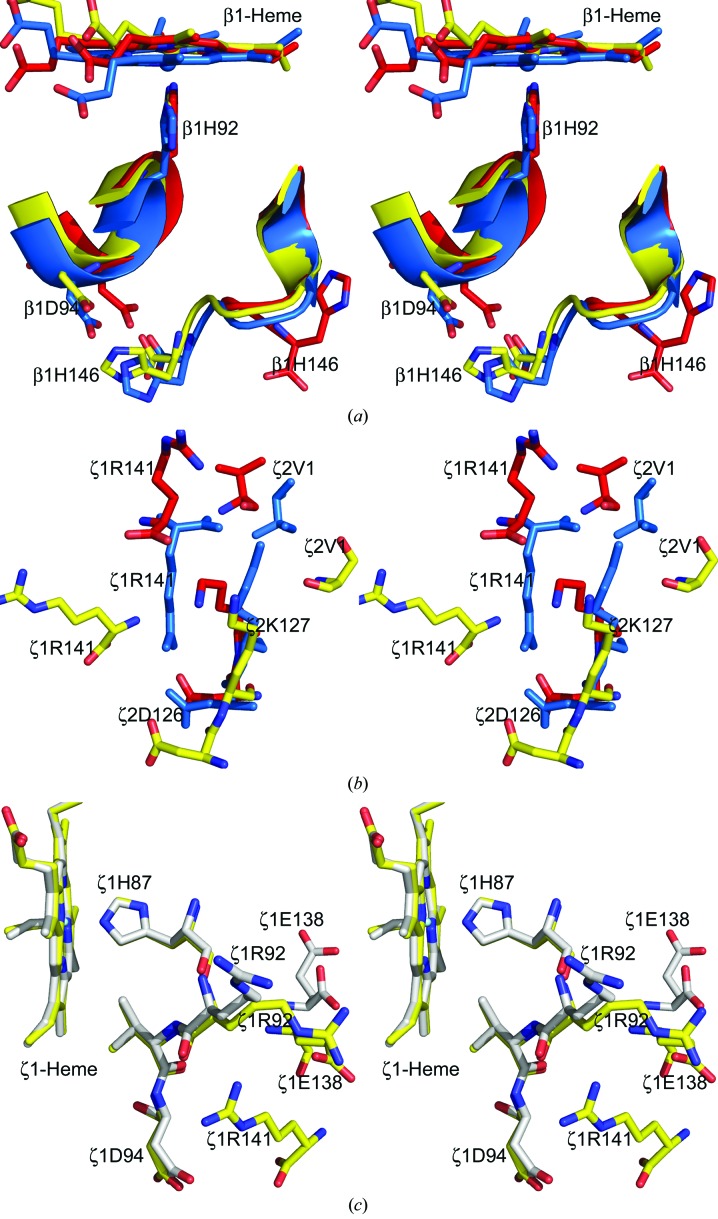
Figure 4.
Stereoview of interactions associated with the α- (ζ-) and β-subunit C-termini in the T (PDB entries 2hhb or 2dn2; cyan), R (PDB entries 1aj9 or 1ljw; red), Hb ζ2β2 s ABCD (yellow) and/or EFGH (gray) structures. (a) The T-state salt-bridge interaction between β1His146 and β1Asp94 is broken in the R structure but is maintained in the liganded Hb ζ2β2 s structure. (b) The T-state salt-bridge interactions between α1Arg141 and α2Lys120 and α2Asp126 are broken in both the R and the liganded Hb ζ2β2 s structures. (c) Reorientation of the Hb ζ2β2 s ABCD ζ-subunit C-terminus results in hydrogen-bond interactions between ζ1Arg141 and the F-helix residues ζ1Arg92, ζ1Asp94 and ζ1Glu138. The corresponding interactions in the EFGH tetramer are missing owing to disorder of the ζArg141 residue.