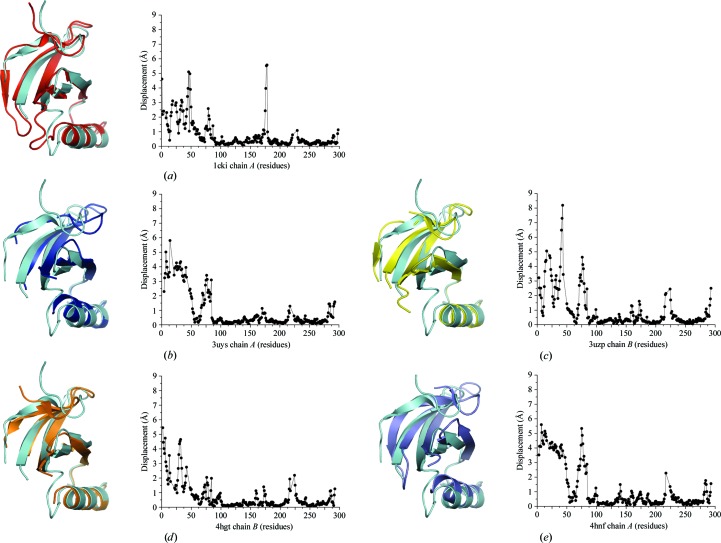
Figure 3.
Conformational differences in the N-terminal lobe. Chains were aligned by the residues of the C-terminal lobe (amino acids 88–281). The C-terminus of helix αA aligns well in all structures, but rotation of the β-sheet, and in some cases pivoting of αA, are observed between different structures. R.m.s.d. values were calculated using Chimera (Pettersen et al., 2004 ▶). (a) Superposition of CK1δ (1–299) chain B (cyan) with 1cki chain A (red). (b) Superposition of CK1δ (1–299) chain B (cyan) with 3uys chain A (blue). (c) Superposition of CK1δ (1–299) chain B (cyan) with 3uzp chain B (yellow). (d) Superposition of CK1δ (1–299) chain B (cyan) with 4hgt chain B (orange). (e) Superposition of CK1δ (1–299) chain B (cyan) with 4hnf chain A (purple).