Figure 4.
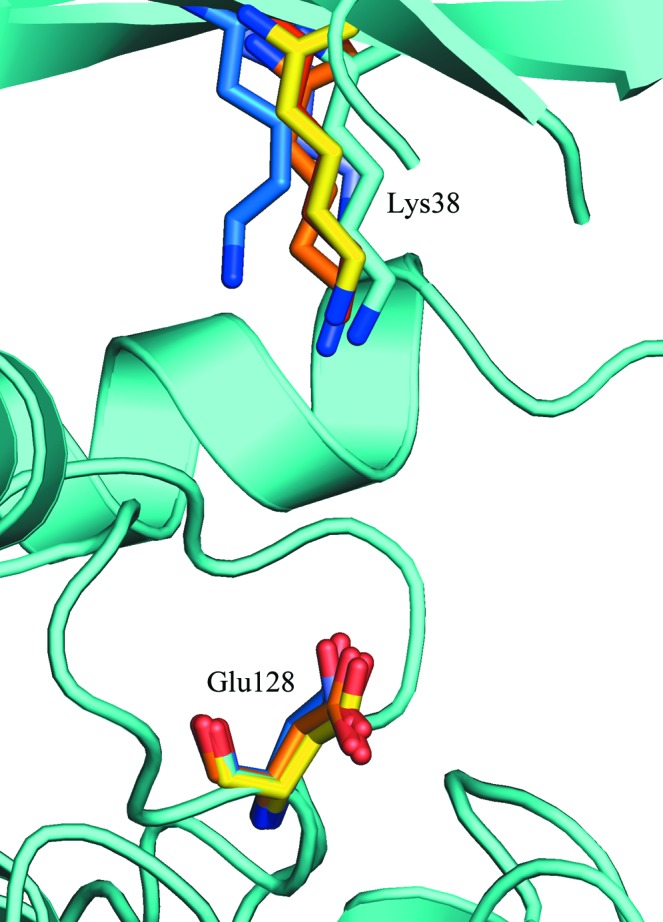
Catalytically important residues. Lys38 and Glu128 from 1cki chain A (red), 3uys chain A (blue), 3uzp chain B (yellow), 4hgt chain B (orange) and 4hnf chain A (purple) are superimposed on the structure of CK1δ (1–299) chain B (cyan) after alignment by the C-terminal lobes as in Fig. 3 ▶. The conformation of the putative general base Glu128 does not change much between structures, but the conformational changes in the β-sheet shown in Fig. 3 ▶ lead to small shifts in the position of the ATP-binding residue Lys38.
