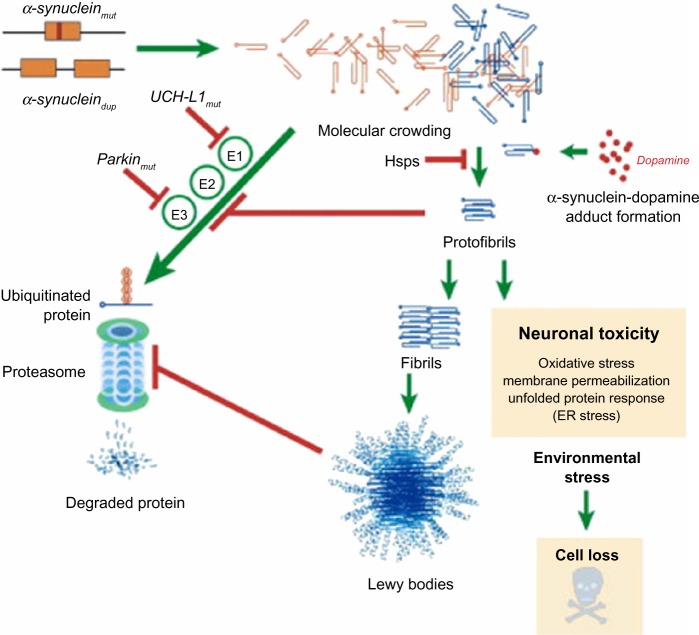
Figure 2.
Putative mechanisms of α-synuclein neurodegeneration in familial PD.
Notes: The presence of Lewy bodies in PD is caused by a failure to properly dispose of α-synuclein. Point mutations in α-synuclein and the recently described Iowan functional duplication lead to excessive intracellular accumulation of α-synuclein, resulting in spontaneous oligomerization of this protein. Mutant α-synuclein can inhibit proteasomal activity, potentially leading to enhanced accumulation; soluble α-synuclein protofibrils and intracellular protein aggregates have been shown to impair the proteasome. Dopamine may also form stable adducts with protofibrils, potentially enhancing this toxicity. Genetic mutations associated with the UPP pathway, including parkin and UCH-L1, are associated with familiar PD. Combined with other cellular stress, this leads to neuronal loss.
Copyright © 2003. Elsevier. Reproduced with permission from Eriksen JL, Dawson TM, Dickson DW, Petrucelli L. Caught in the act: α-synuclein is the culprit in Parkinson’s disease. Neuron. 2003;40(3):453–456.29
Abbreviations: PD, Parkinson’s disease; UCH-L1, ubiquitin carboxy-terminal hydrolase L1; UPP, ubiquitin proteasome pathway.