Abstract
Acute Respiratory Failure patients experience significant muscle weakness which contributes to prolonged hospitalization and functional impairments post-hospital discharge. Based on our previous work, we hypothesize that an exercise intervention initiated early in the intensive care unit aimed at improving skeletal muscle strength could decrease hospital stay and attenuate the deconditioning and skeletal muscle weakness experienced by these patients.
Summary
Early exercise has the potential to decrease hospital length of stay and improve function in Acute Respiratory Failure patients.
Keywords: Length of Stay, Physical Function, Mobility, Intensive Care Unit, Strength Training
Introduction
Exercise interventions have led to impressive gains in the management of chronic diseases (13). Clinical data from our outpatient investigations has increased our appreciation of exercise’s potential within the inpatient setting (5; 7; 8; 19; 36; 40). In this era of increasing hospital costs, the use of early inpatient exercise training may represent a safe and cost-effective alternative for hospitals, particularly in the early application of mobility and exercise interventions within the intensive care unit (ICU). Exercise training has the potential to favorably modify the length of ICU and hospital stay and the level of physical function in critically ill patients. ICU patients afflicted with acute respiratory failure (ARF) commonly experience long ICU and hospital stays and a constellation of limitations, including reductions in cardiorespiratory fitness, loss of skeletal muscle mass and strength, neuropathy, pain, depression, delirium and anxiety. The success of exercise training in the management of patients with a chronic disease has provided an impetus to study the potential of early inpatient exercise interventions for the rehabilitation for ICU patients.
In this article, under the heading “Acute Respiratory Failure”, we will examine the epidemiology of ARF and the consequences of this disorder to both society and the individual patient. Under the heading “Skeletal Weakness in ARF Patients”, specific emphasis will be placed on the loss of skeletal muscle strength following an ICU stay and the potential mechanisms responsible for this loss. Under the heading of “Rehabilitation Of Skeletal Muscle Weakness”, we will examine the role of exercise at improving physical function in patients experiencing similar physical manifestations as those of ARF patients and will examine studies providing evidence for the use of an early exercise intervention in the treatment of these patients. Finally, under the heading of “Rehabilitation of the ARF Patient” we will propose strength training as an intervention to decrease hospital and ICU length of stay and to improve physical function in ARF patients and will offer insights as to how best initiate such a program in an ICU.
Acute Respiratory Failure (ARF)
Epidemiology
ARF is a heterogeneous syndrome defined as an acute cardiopulmonary dysfunction requiring emergent artificial ventilation support whether it be invasive or non-invasive. It may be neuromuscular in origin; secondary to acute or chronic obstructive pulmonary disease; a result of alveolar disruptions due to pulmonary edema, pneumonia or interstitial diseases; or a result of a vascular disease such as acute or chronic pulmonary embolism (30). Of patients admitted to an ICU, over 30% are admitted with ARF, and over half of all ICU patients will suffer ARF sometime during their stay (48). In a recent report, the in-ICU mortality was more than double for those patients with ARF versus those without (48). For those surviving, the average ICU length of stay for ARF patients is 50 percent longer than those without (48). ARF patients incur significantly higher daily costs for ICU treatment versus non-ARF ICU patients, as the incremental cost of receiving mechanical ventilation is greater than $1,500 per day (14). As such, interventions targeting a reduced ICU stay and/or duration of mechanical ventilation could lead to substantial reductions in hospital costs.
Skeletal Weakness in ARF Patients
ICU Acquired Weakness
In addition to increased lengths of stay, mortality rates and hospital costs, ARF patients are at an increased risk to develop ICU acquired skeletal muscle weakness which results in an increased risk of morbidity (15) and significant functional impairments post-hospital discharge (28; 29). ICU acquired skeletal muscle weakness is thought to result from neuropathies of the somatic motor neurons serving skeletal muscle (critical illness polyneuropathy), myopathies of the skeletal muscle (critical illness myopathy) or a combination of the two (critical illness neuromyopathy). Regardless of the etiology, skeletal muscle weakness is a common characteristic of ARF patients which contributes to prolonged hospitalization, the inability to perform activities of daily living, a reduced exercise capacity and the need for extended care (28). Skeletal muscle weakness has been reported in 25 to 33% of ARF patients who have been ventilated from four to seven days (15) and is associated with increased mortality (1). Skeletal muscle weakness in ARF survivors has been shown to persist up to one year after ICU discharge such that the six minute walk distance for these patients was 66% of predicted for age- and sex-matched healthy controls (28). In a prospective cohort study of 1,075 ARF survivors at five months post-discharge, 48 percent needed help with at least one activity of daily living and 24 percent reported needing assistance with more activities of daily living as compared to pre-hospitalization (25). In one study of ARF survivors with a prolonged ICU stay, skeletal muscle weakness was reported as long as 3.5 years following discharge (21). In a follow-up study of survivors of acute respiratory distress syndrome (a subpopulation of ARF patients), a high prevalence of persistent skeletal muscle weakness and fatigue was reported one year later (28). At five years post-ICU discharge, these same patients presented with a reduced exercise capacity and reported varying degrees of perceived weakness (29). These data illustrate the deleterious effects ARF has on patient function post-hospital discharge. Additionally, they suggest a need for interventions that could shorten ICU or hospital stay and improve physical function. Such interventions could offer healthcare systems cost reductions while simultaneously providing an improvement measurable on a patient-specific level. We will present data from our previous work to suggest that ICU-associated strength training may be such an intervention.
Clinical Assessment of ARF Patients
The clinical assessment of skeletal muscle weakness in ARF patients may be determined in a number of ways. For most research studies, the direct measurement of muscle strength is typically performed using manual muscle testing with the Medical Research Council scoring algorithm or with a hand held dynamometer (9; 20). Both methods have been used in studies conducted in the ICU and have good reliability. A disadvantage of these techniques is they are often not only limited by the patient’s ability to cooperate, but by positioning, pain, edema, effort level and fatigue. Additionally, they provide little information on the underlying cause of the muscle weakness (47).
To confirm a diagnosis of critical illness polyneuropathy, electrophysiological studies that evaluate peripheral nervous function are needed. These assessments include nerve conduction studies, electromyography and neuromuscular junction testing. While these tests can help identify the underlying etiology, they require dedicated equipment and trained personnel. In addition, some of these techniques may be painful and require patient cooperation. The analysis of muscle biopsies obtained from ARF patients can be used to diagnose critical illness myopathy and has provided some insight as to the mechanisms underlying ICU acquired skeletal muscle weakness and wasting. These mechanisms include the loss of skeletal muscle myosin fibers and atrophy of type II muscle fibers (47). As with the assessment of peripheral nerve function, dedicated equipment and trained personnel are needed. In addition these studies are invasive and can be painful.
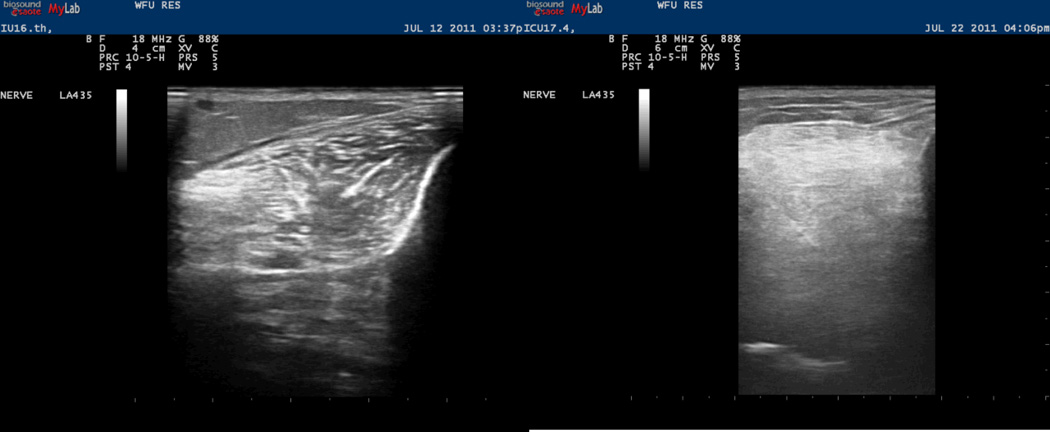
Because of the limitations in using electrophysiological studies and muscle biopsies to support the diagnosis of ICU acquired weakness, new techniques to assist in the diagnosis and serve as markers of disease progression are currently being explored. The use of ultrasound techniques has been recently suggested as a technique to diagnose and monitor the progress of ICU acquired weakness (47). Using high resolution ultrasound imaging, we have shown that muscle echotexture changes of the lower extremity and diaphragm muscles of ICU patients are similar to those seen in patients with muscular dystrophies and chronic inflammatory myopathies (11). These changes include muscle degradation and the loss of the normally well-organized muscle architecture that can occur during a stay in the ICU (Figure 1). Further studies are needed to validate the use of this technique for diagnosing and monitoring the progress of skeletal muscle weakness in the patients.
Figure 1.
The image on the left shows the tibialis anterior muscle in cross-section at baseline. The image on the right shows the same tibialis anterior muscle after the patient has been in the ICU for 14 days. After the prolonged ICU stay the muscle has lost its normal echotexture and is hyperechoic with a homogeneous, ground-glass appearance. (Reprinted from (11). Copyright © 2012 John Wiley and Sons. Used with permission.)
Risk Factors for ICU Acquired Weakness
For ARF patients, the ICU acquired skeletal muscle weakness is a complex and poorly understood pathophysiological disorder. A number of factors have been cited for the loss of strength and physical function; however, the interaction among the various factors has not been well defined. Predisposing factors associated with this disorder are presented in Table 1. Whatever the cause, the resultant clinical picture is significant skeletal muscle weakness following discharge which may persist for months or even years (29).
Table 1.
Predisposing Factors Associated with Critical Illness Polyneuropathy and Critical Illness Myopathy
| Critical Illness Polyneuropathy |
| Sepsis |
| Systemic Inflammatory Response |
| Multi-Organ Failure |
| Critical Illness Myopathy |
| Asthma |
| Exacerbation of Chronic Obstructive |
| Pulmonary Disease |
| Acute Respiratory Distress Syndrome |
| Corticosteroid Use |
| Neuromuscular Blocking Agent Use |
Pharmacological Agents
Skeletal muscle weakness may be accentuated by the use of ICU medications such as corticosteroids and neuromuscular blocking agents (16; 37). ICU patients can be exposed to high doses of these drugs for prolonged periods. While the effects of corticosteroids on skeletal muscle have been studied, it is difficult to separate the effect of these drugs from those of the underlying disease and the interaction among the other pharmacological treatments used in the ICU. Nava and colleagues studied the effects of high doses of methylprednisolone in the absence of other pharmacological therapy over five days in 13 transplant patients with acute lung rejection. They found that compared to the pretreatment condition, approximately 45% of patients showed generalized muscle weakness following treatment (37). When investigating the effects of corticosteroids in animal models, investigators have found a steroid-induced myopathy to occur in primarily fast-twitch (type II) fibers as compared to slow-twitch (type I) fibers. Additionally, it has been shown that immobilization exacerbates corticosteroid-induced skeletal muscle atrophy, whereas remobilization of a previously immobilized muscle prevents further corticosteroid-induced atrophy (43). While the use of neuromuscular blocking agents has decreased in ARF patients, the effects of these drugs and their interactions with other drugs administered in the ICU are poorly understood (43). There are numerous case reports and retrospective studies documenting skeletal muscle weakness following the use of these agents (16; 32; 37); however, the adverse relationship between neuromuscular blocking agents and skeletal muscle function following their administration has recently been challenged (43).
Deconditioning
Described as the changes in organ system physiology induced by immobilization, deconditioning is believed to be reversed by physical activity. In the clinical setting, acute deconditioning refers to changes that occur within days to a few weeks of a sudden decrease in activity, and concern regarding bed rest in hospitalized patients is not new (34). Currently, admission to an ICU implies almost certain imposed immobility, particularly when mechanical ventilation is required. In numerous reports on healthy subjects, it has been well demonstrated that immobilization induces skeletal muscle atrophy which is characterized by a decrease in protein content, fiber diameter, fatigue resistance and force production. These changes appear to be mediated primarily by a down regulation of protein synthesis that occurs early in the immobilization period (43).
Age
An additional factor effecting outcomes in ARF patients is age which, although controversial, has been shown by some to be an independent predictor of mortality and impaired physical function (25; 48). Aging can compound the effect that ARF has on physical functioning due to the independent effect it has on skeletal muscle and strength. Aging in healthy older adults is associated with a significant loss of skeletal muscle and strength which have been shown to be associated with a decline in physical function and disability (38). An analysis of adults aged 60 and older showed the likelihood of functional impairment and disability to be approximately two to three times greater in those with severe muscle loss (17). In a subsequent 8-year follow-up, the risk of developing disability was 27% greater in those with severe muscle loss compared to those with normal muscle mass (17). Thus older adults who experience ARF are at increased risk for disability and a loss of strength and physical function.
Sepsis and Systemic Inflammation
Sepsis, a condition whereby the body has an overwhelming immune response to infection, leads to systemic inflammation and may also contribute to ICU weakness. Sepsis may be the most commonly experienced exposure to systemic inflammation for ARF patients (18). Systemic inflammation may further exacerbate the loss of muscle protein and muscle function. Studies suggest that inflammatory mediators inhibit muscle function, although the mechanisms by which this occurs are not fully understood (12). The effects of pro-inflammatory cytokines on muscle have been studied in a variety of muscle wasting conditions, and all have demonstrated an association with loss of muscle mass (12). We examined the relationships between physical function and inflammatory biomarkers in 542 older men and women who had chronic obstructive pulmonary disease, congestive heart failure, high cardiovascular risk, or self-reported physical disability. We found that elevated levels of the pro-inflammatory cytokines C reactive protein and interleukin 6 were associated with poorer physical function in older adults with these various conditions (10). An exacerbation of any of these chronic subclinical inflammatory conditions could result in an ICU admission and, thus, has the potential to further limit physical function in older persons with a pre-existing disease or health condition.
It should be noted that exact role of cytokines during inflammation has yet to be clearly defined. While the cytokine storm seen in critically ill patients is overall myopathic, there are data to suggest increases in cytokines can be beneficial as they play a role in protein synthesis (46) and pain management (31). The relationships between cytokines and protein synthesis are complex; it is well established that they play a significant role in muscle fiber growth (46), but are also associated with muscle wasting (27).
Rehabilitation Of Skeletal Muscle Weakness
The etiology of skeletal muscle weakness associated with an ICU stay is likely mutifactorial. Whatever the primary etiology, skeletal muscle weakness has been shown to be associated with deleterious short- and long-term outcomes (28; 29). Consequently, the use of exercise and early mobilization has been suggested as adjunctive therapy in the early treatment of these patients (4; 26). The use of exercise as a treatment of other chronic conditions associated with skeletal muscle weakness has been more fully investigated, and the general consensus is that exercise is an effective treatment for secondary skeletal myopathies and the associated weakness. This is especially true in pulmonary (6–8), cardiovascular (42) and geriatric medicine (19).
Exercise and Chronic Diseases
Chronic diseases such as heart failure and chronic obstructive pulmonary disease (COPD) are known to result in skeletal muscle weakness (2; 42). Similar to ICU acquired muscular weakness, the etiology of weakness in heart failure and COPD is believed to be multi-factorial. It may be the result of deconditioning, age, medications or inflammation. We have previously shown that participation in an aerobic exercise training program results in significant improvements in physical function for COPD patients independent of improvements in lung or cardiovascular function (5–7). These results suggest that skeletal muscle and the nervous system are the organs responsive to exercise training (2). Skeletal muscle dysfunction is now recognized as a major problem for COPD patients (2), and, as such, the use of strength training as a modality to reverse the deleterious effects of the disease is now recommended (39). A recent systematic review demonstrated strength training could improve skeletal muscle strength in COPD patients, and these improvements were associated with increased activities of daily living (41).
As with COPD patients, heart failure patients present with reduced skeletal muscle mass and strength. Although most clinical studies have examined the effects of aerobic exercise training on physical function and performance outcomes for heart failure patients, a growing body of information supports strength training as an additional intervention (42). Results from the above literature (2; 42) suggests that exercise training, in particular strength training, may be beneficial for patients with skeletal muscle weakness, and provides a strong rationale for investigations within the ICU examining the efficacy of strength training interventions for ARF patients.
Exercise intervention studies in chronic diseases such as heart failure and frailty of aging have demonstrated associations among exercise, reductions in pro-inflammatory cytokines and improvements in physical function. The sequence of cytokine elaboration in the blood during exercise has characteristics similar to the “resolution” phase of acute inflammation. Interestingly, with acute moderate exercise, the classic pro-inflammatory cytokines TNF and IL-1 do not increase, whereas, IL-6 concentrations do. Other anti-inflammatory cytokines such as IL-10, Interleukin 1 receptor antagonist and soluble TNF receptors are found to rise in response to exercise (49). Because of the similarities seen between the cytokine pattern of exercise and the “resolution” phase of acute inflammation, some have speculated that exercise has therapeutic potential in acute inflammatory conditions (49).
The effect of strength training on the progress of glucocorticoid-induced atrophy has also been investigated. Gardiner et al. showed that a strength training program can decrease the atrophy and strength loss associated with steroid use. They also demonstrated that the extent of fast-twitch muscle atrophy resulting from chronic glucocorticoid treatment can be lessened by mild weight-lifting exercise (24).
Based on the results of previous investigations examining the role of exercise in the treatment of chronic conditions, collaborations between critical care clinicians and exercise specialist examining the use of early exercise in the rehabilitation of ARF patients may produce similar results.
Rehabilitation of the ARF Patient
In-hospital exercise intervention
Despite the evidence to suggest that exercise may play a significant role in the treatment of chronic diseases resulting in skeletal muscle weakness, the role of exercise in facilitating a return to pre-hospital functional status for ARF patients and the exact manner by which to prescribe ICU and hospital exercise has yet to be determined. To help determine the best approach to prescribing exercise in this patient population, we have assembled a team of professionals that consist of physicians certified in the subspecialty of Critical Care Medicine, nurses, physical therapists and clinical exercise physiologists. While the physicians and nurses are responsible for the medical management of these patients, the physical therapist and clinical exercise physiologist work together to determine the specific rehabilitation needs of individual patients. Clinical exercise physiologists are trained to identify specific deficits and are knowledgeable as to the modality or type of exercise that can be best utilized based on a patient’s specific needs (22). They are also cognizant of the frequency with which the exercise should be performed, the intensity of the exercise and the duration or time of the exercise. The physical therapists often define the best approach to increasing mobility in these patients. We therefore recommend that the clinical exercise physiologist and physical therapist work together in developing an individualized rehabilitation plan for ICU patients.
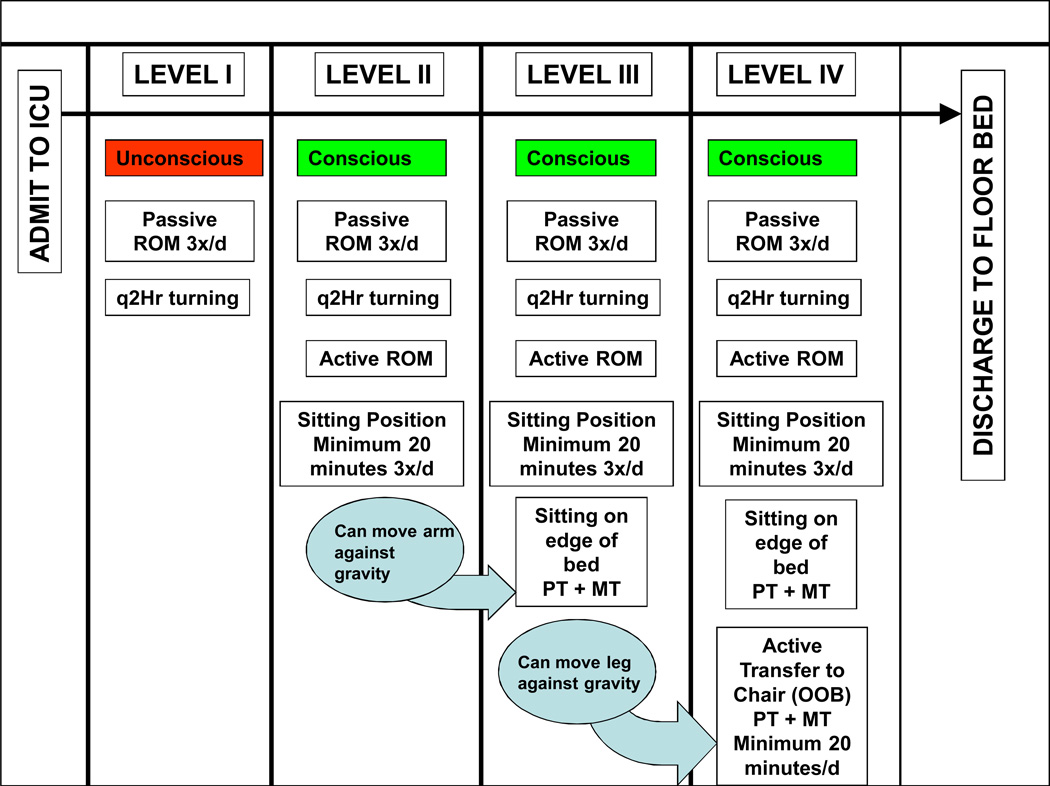
Our team has developed an approach for delivering exercise and rehabilitation therapy early in an ICU stay to ARF patients. To do so, we enrolled 330 ICU patients with ARF into a prospective cohort study to determine whether a protocol emphasizing early exercise and rehabilitation increased the proportion of ICU patients receiving exercise and physical therapy (n = 165) versus usual care (n = 165). The early exercise and rehabilitation protocol consisted of increasing levels of physical activity which included both passive and active range of motion (ROM) exercises and physical therapy aimed at increasing a patient’s mobility. Figure 2 illustrates the protocol we used to initiate exercise in the ICU. Of survivors, this study showed that a greater percentage of protocol patients received at least one physical therapy session as compared to usual care patients (80% versus 47%, respectively; p = 0.001). Protocol patients were out of bed earlier (5 vs. 11 days, p=<0.001), had therapy initiated more frequently in the ICU (91% vs. 13%, p=<0.001), and had no difference in ICU complication rates compared to usual care. Among survivors, for protocol patients, ICU length of stay was 5.5 vs. 6.9 days for usual care (p = 0.025); hospital length of stay for protocol patients was 11.2 vs. 14.5 days for usual care (p = 0.006). In this investigation, we demonstrated both the feasibility and safety of an early exercise and physical therapy intervention. Additionally, this study demonstrated that exercise and physical therapy initiated early in an ICU stay is associated with important outcomes and has the potential to reduce both ICU and hospital length of stay (35).
Figure 2.
Passive range of motion (ROM) therapy started on day 1 of protocol (level I). As patients demonstrated consciousness and increased strength, they were moved to the next higher level. Physical therapy (PT) would be first attempted at level II. The protocol’s intervention ceased as a patient was transferred to a bed in a general care area, and then patients within both “protocol” and “usual care” groups would receive usual care mobility therapy (MT) as dictated by the physician teams in the general care areas. ICU, intensive care unit; OOB ,out of bed; q2Hr, every 2 hours; 3x/d, 3 times a day. (Reprinted from (35). Copyright © 2008. Wolters Kluwer Health. Used with permission.)
We subsequently identified a cohort of survivors who had participated in our earlier trial that required subsequent hospitalization. We used multiple logistic regression to identify variables that would predict hospital readmission or death in the first year following discharge from the patient’s initial hospitalization. The lack of early exercise and rehabilitation therapy was found to be a significant predictor of hospital readmission or death (36). These data suggest that early exercise and rehabilitation therapy may reduce subsequent hospitalizations and provide further support for the use of exercise therapy early in an ICU visit.
Despite these positive reports showing benefits from ICU initiated exercise therapies, no current consensus statements exist for the timing or content of ICU or hospital exercise strategies for ARF patients. Although a European consensus statement on exercise therapy in the ICU called for research, the authors were not able to recommend any one exercise intervention for ARF patients due to the lack of strong scientific evidence (26). There is a clear need to establish a standard for practice, to prioritize the use of exercise in the in-hospital rehabilitation of ARF patients and to determine the optimal training program for these patients such that improvements in functional status occur. Based on our results using exercise to improve physical function in patients with chronic diseases and with using early mobility interventions to decrease ICU and hospital lengths of stay in ARF patients, we hypothesize that an exercise intervention initiated early in an ARF patients ICU stay and targeting improvements in skeletal muscle function would not only reduce length of stay, but it would also improve physical function post discharge.
To initiate such a program, collaborations between physicians and nurses charged with the care of patients in the ICU and exercise specialist would be needed. The ICU environment presents unique challenges to the exercise specialist. These include such things as safety concerns for the patient, multiple vascular access devices, patient sedation and tight space confines (34). Strong collaborations with clinicians will allow the exercise specialist to provide a focused approach to prescribing exercise to the patient with an eye on challenging the patient such that they could progress at a maximal rate.
Despite the lack of consensus on the optimal rehabilitation therapy for ARF patients, the research demonstrating a profound weakness in these patients due to neuropathies and myopathies suggest that strength training may be a type of exercise intervention of considerable benefit for the ARF patient. Strength training results in both neurological and muscle structural improvements. Studies have shown that the increases in strength seen during the early phases of strength training are due to mainly neural adaptations. Subsequent increases result from hypertrophy of individual myocytes (33; 44).
The Strength Training Exercise Prescription
While there is no clear consensus as to the optimal training prescription for ARF patients, the recommendations from the American College of Sports Medicine (ACSM) provide a starting point (23). To optimize the benefits gleaned from a strength training program, the exercise prescription variables of intensity, volume and frequency should be tailored to the needs of each patient.
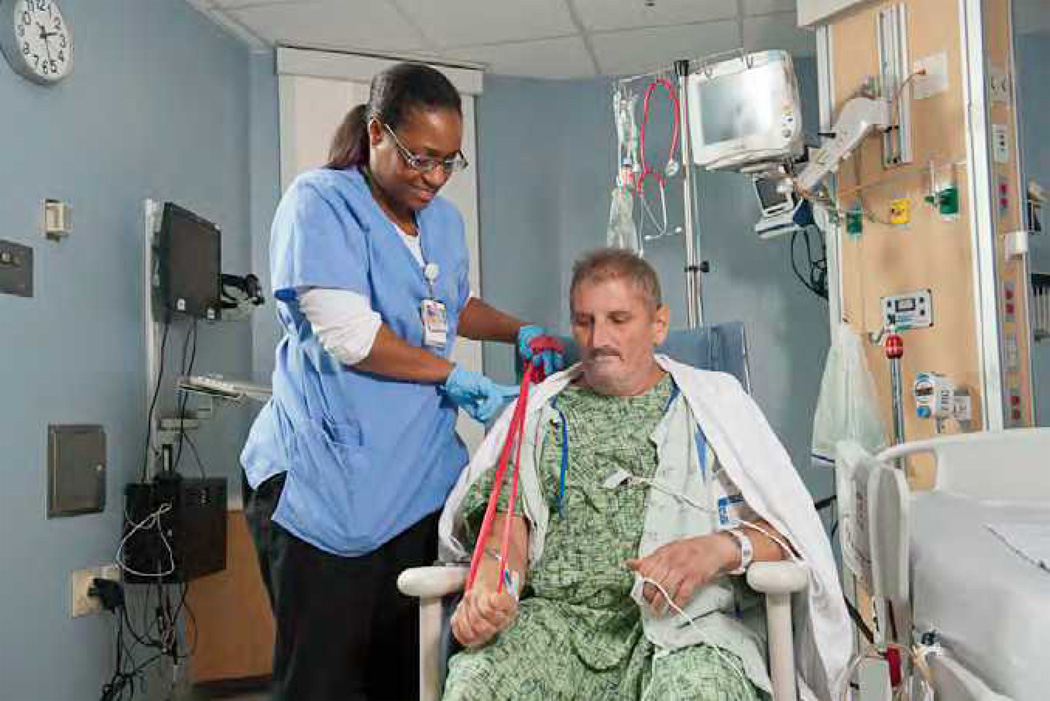
Intensity - Specifically, with extremely frail individuals, the ACSM recommends that strength training exercises be performed at an intensity of approximately 60% of the muscle’s maximum force generating capacity to increase strength. For some critically ill patients, this stimulus may simply consist of moving their limbs against gravity. For others, the stimulus may be significantly greater despite the ongoing need of mechanical ventilation and ICU care. To determine the amount of weight of the appropriate stimulus, the common outpatient approach is based on a 1 repetition maximum, (1-RM) or the ability to lift or perform a movement only once before fatigue prevents a subsequent contraction. If the weight employed is below 60% of the 1-RM, there may be only modest improvements of 5% to 10% in strength, with the observed improvement likely due to a neural adaptations rather than an increase in contractile protein in the muscle. Because of the obvious problems associated with determining the 1-RM in the ICU, an alternative approach is to have patients perform 8 to 12 maximum repetitions of a given exercise. If the patient is fatigued and unable to perform any additional repetitions after 8 to 12 repetitions, the patient is working at appropriate intensity. Resistance may be applied by using hand held or strap on weights. Another alternative is the use of elastic hoses or bands. Figure 3 shows an ARF patient performing strength training exercises with an elastic band.
Figure 3.
Acute Respiratory Failure patient performing elbow extension exercises with elastic band.
Volume - The volume of the strength training exercise is a function of the number of repetitions and sets of an exercise completed. Most individuals can realize strength gains with two to four sets of strength exercises per muscle group. For frail patients, the ACSM recommends that at least one set of each exercise be performed. As patients increase the intensity of their exercise prescription, the number of sets of an exercise may also increase. Once the patient can complete the pre-determined number of repetitions for all the prescribed sets, the resistance should be increased. The duration or time of the exercise bout will vary depending on the number of specific exercises performed along with the number of sets and repetitions. Specific exercises should target strength improvements for the major muscle groups which include the chest, shoulders, arms, upper and lower back, abdomen, hips and legs. One or more exercises can be prescribed for each of these muscle groups depending upon the patient’s specific needs.
Frequency - The final component of the exercise prescription that needs to be considered is frequency or the number of times the exercise should be performed per week. Outpatient recommendations stipulate that individuals exercise 2 to 3 times per week and to allow 48 hours of recovery between exercise bouts. The optimal frequency of early strength training interventions for ARF patients is yet to be determined and may be more frequent. Another consideration for prescribing strength training to ARF patients is when such a program should be initiated. We recommend initiating a strength training program as soon as the patient is able to perform 8 to 10 repetitions of active range of motion exercises. This typically coincides with obtaining consciousness (Figure 2). The above recommendations are based on ACSMs guidelines for exercise prescription which are supported by a substantial volume of research that has been conducted with healthy, younger and older populations and some diseased populations (23).
A final and important concern when exercising patients in the ICU is safety. Since these subjects are critically ill, require mechanical ventilation, and often have serious underlying illnesses, complications are unfortunately common in this population. Risks possibly associated with exercising patients in the ICU could include hypoxemia, cardiac arrhythmias, falls, hypertensive response or the loss of a management device such as an endotracheal tube or vascular access. In previous investigations examining the efficacy of early rehabilitation in ICU patients, there were no deaths, near-deaths, cardiopulmonary resuscitation events or extubations occurring during rehabilitation sessions (3; 35; 45). Additionally, only two accidental removals of devices during exercise were reported, a radial artery catheter (45) and a feeding tube (3). Five falls to the knees without injury were reported by Bailey (3). There were no complications resulting from rehabilitation that required additional therapy, additional cost, or longer length of hospital stays reported in any of the three studies (3; 35; 45). These results show that early exercise with ARF patients is safe as long as there is coordinated effort from a multi-disciplinary team consisting of physicians, nurses, physical therapists and exercise specialists
Conclusion
ARF patients present with a constellation of problems including significant skeletal muscle weakness as a result of myriad factors. Research on other patient populations with skeletal muscle weakness provide strong evidence for the use of strength training in ARF patients. Unfortunately, there is little research on exercise training and rehabilitation of ARF patients and none on how best to prescribe exercise in the ICU setting. The outpatient approach to exercise prescription development does however provide a framework upon which ICU exercise prescriptions may be modeled. Hopefully, fruitful collaborations between ICU specialists, nurses, physical therapists and exercise physiologists will provide the needed evidence for specific recommendations regarding exercise as a rehabilitation tool for ICU patients.
Acknowledgments
This work was supported by the National Institutes of Health [Grants NR011186, HL53755]; and Wake Forest University Claude D. Pepper Older Americans Independence Center [P30-AG21332].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ali NA, O'Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, Connors AF, Jr, Marsh CB. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society European Respiratory Society. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:S1–S40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 3.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, Veale K, Rodriquez L, Hopkins RO. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 4.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 5.Berry MJ, Adair NE, Sevensky KS, Quinby A, Lever HM. Inspiratory muscle training and whole-body reconditioning in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:1812–1816. doi: 10.1164/ajrccm.153.6.8665039. [DOI] [PubMed] [Google Scholar]
- 6.Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Jr, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Berry MJ, Rejeski WJ, Adair NE, Zaccaro D. Exercise rehabilitation and chronic obstructive pulmonary disease stage. Am J Respir Crit Care Med. 1999;160:1248–1253. doi: 10.1164/ajrccm.160.4.9901014. [DOI] [PubMed] [Google Scholar]
- 8.Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, Katula JA. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med. 2010;104:829–839. doi: 10.1016/j.rmed.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon RW. Manual muscle test scores and dynamometer test scores of knee extension strength. Arch Phys Med Rehabil. 1986;67:390–392. [PubMed] [Google Scholar]
- 10.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartwright MS, Kwayisi G, Griffin LP, Sarwal A, Walker FO, Harris JH, Berry MJ, Chahal PS, Morris PE. Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve. 2013;47:255–259. doi: 10.1002/mus.23525. [DOI] [PubMed] [Google Scholar]
- 12.Chang HR, Bistrian B. The role of cytokines in the catabolic consequences of infection and injury. JPEN J Parenter Enteral Nutr. 1998;22:156–166. doi: 10.1177/0148607198022003156. [DOI] [PubMed] [Google Scholar]
- 13.Charansonney OL. Physical activity and aging: a life-long story. Discov Med. 2011;12:177–185. [PubMed] [Google Scholar]
- 14.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 15.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 16.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 17.Di IA, Abate M, Di RD, Russolillo A, Battaglini C, Ripari P, Saggini R, Paganelli R, Abate G. Sarcopenia: age-related skeletal muscle changes from determinants to physical disability. Int J Immunopathol Pharmacol. 2006;19:703–719. doi: 10.1177/039463200601900401. [DOI] [PubMed] [Google Scholar]
- 18.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger WHJ, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, Shumaker S, Berry MJ, O'Toole M, Monu J, Craven T. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis The Fitness Arthritis and Seniors Trial (FAST) J Am Med Assoc. 1997;277:25–31. [PubMed] [Google Scholar]
- 20.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36:1038–1043. doi: 10.1007/s00134-010-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016. doi: 10.1097/01.CCM.0000053651.38421.D9. [DOI] [PubMed] [Google Scholar]
- 22.Franklin B, Fern A, Fowler A, Spring T, Dejong A. Exercise physiologist's role in clinical practice. Br J Sports Med. 2009;43:93–98. doi: 10.1136/bjsm.2008.055202. [DOI] [PubMed] [Google Scholar]
- 23.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 24.Gardiner PF, Hibl B, Simpson DR, Roy R, Edgerton VR. Effects of a mild weight-lifting program on the progress of glucocorticoid-induced atrophy in rat hindlimb muscles. Pflugers Arch. 1980;385:147–153. doi: 10.1007/BF00588695. [DOI] [PubMed] [Google Scholar]
- 25.Garland A, Dawson NV, Altmann I, Thomas CL, Phillips RS, Tsevat J, Desbiens NA, Bellamy PE, Knaus WA, Connors AF., Jr Outcomes up to 5 years after severe, acute respiratory failure. Chest. 2004;126:1897–1904. doi: 10.1378/chest.126.6.1897. [DOI] [PubMed] [Google Scholar]
- 26.Gosselink R, Bott J, Johnson M, Dean E, Nava S, Norrenberg M, Schonhofer B, Stiller K, van de Leur H, Vincent JL. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med. 2008;34:1188–1199. doi: 10.1007/s00134-008-1026-7. [DOI] [PubMed] [Google Scholar]
- 27.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 28.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, az-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 29.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 30.Mac Sweeney R, McAuley DF, Matthay MA. Acute lung failure. Semin Respir Crit Care Med. 2011;32:607–625. doi: 10.1055/s-0031-1287870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer KC, Prielipp RC, Grossman JE, Coursin DB. Prolonged weakness after infusion of atracurium in two intensive care unit patients. Anesth Analg. 1994;78:772–774. doi: 10.1213/00000539-199404000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Moritani T, deVries HA. Potential for gross muscle hypertrophy in older men. J Gerontol. 1980;35:672–682. doi: 10.1093/geronj/35.5.672. [DOI] [PubMed] [Google Scholar]
- 34.Morris PE. Moving our critically ill patients: mobility barriers and benefits. Crit Care Clin. 2007;23:1–20. doi: 10.1016/j.ccc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, Penley L, Howard A, Dixon L, Leach S, Small R, Hite RD, Haponik E. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 36.Morris PE, Griffin L, Berry M, Thompson C, Hite RD, Winkelman C, Hopkins RO, Ross A, Dixon L, Leach S, Haponik E. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341:373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nava S, Fracchia C, Callegari G, Ambrosino N, Barbarito N, Felicetti G. Weakness of respiratory and skeletal muscles after a short course of steroids in patients with acute lung rejection. Eur Respir J. 2002;20:497–499. doi: 10.1183/09031936.02.01732002. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 39.Nici L, Donner C, Wouters E, ZuWallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, Garvey C, Goldstein R, Gosselink R, Lareau S, MacIntyre N, Maltais F, Morgan M, O'Donnell D, Prefault C, Reardon J, Rochester C, Schols A, Singh S, Troosters T. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 40.Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Shea SD, Taylor NF, Paratz J. Peripheral muscle strength training in COPD: a systematic review. Chest. 2004;126:903–914. doi: 10.1378/chest.126.3.903. [DOI] [PubMed] [Google Scholar]
- 42.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 43.Puthucheary Z, Rawal J, Ratnayake G, Harridge S, Montgomery H, Hart N. Neuromuscular blockade and skeletal muscle weakness in critically ill patients: time to rethink the evidence? Am J Respir Crit Care Med. 2012;185:911–917. doi: 10.1164/rccm.201107-1320OE. [DOI] [PubMed] [Google Scholar]
- 44.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20:S135–S145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- 45.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Stevens RD, Hart N, De JB, Sharshar T. Weakness in the ICU: a call to action. Crit Care. 2009;13:1002. doi: 10.1186/cc8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincent JL, Akca S, De MA, Haji-Michael P, Sprung C, Moreno R, Antonelli M, Suter PM. The epidemiology of acute respiratory failure in critically ill patients. Chest. 2002;121:1602–1609. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 49.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]