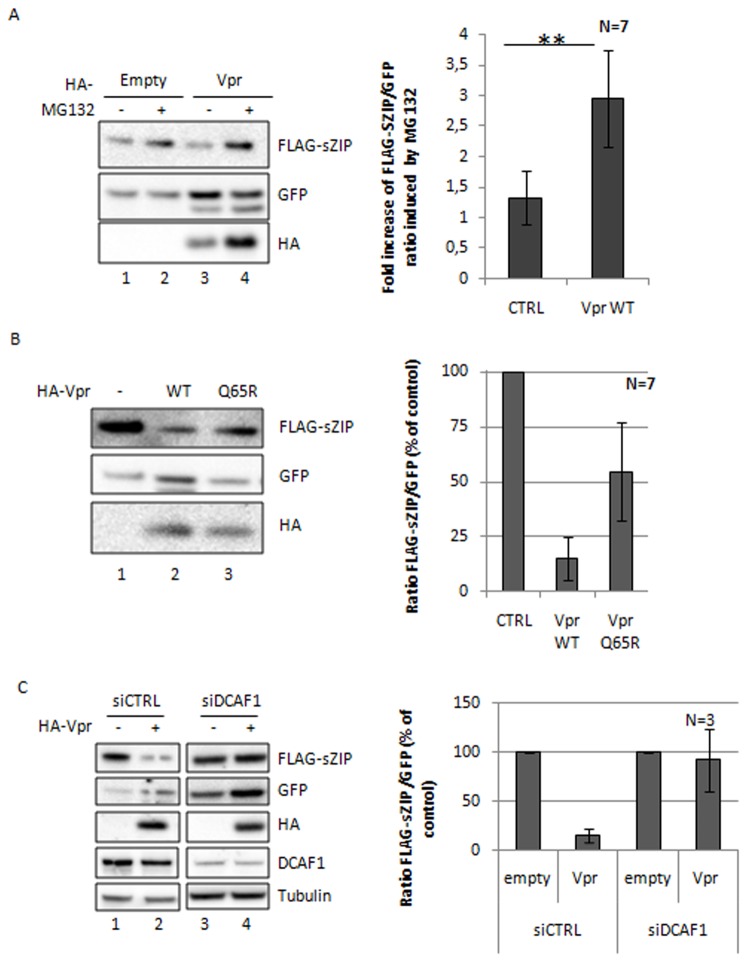
Figure 3. HIV-1 Vpr induces the degradation of sZIP through the DCAF1 ubiquitin ligase.
A. Vpr-mediated sZIP degradation is dependent on the proteasome activity. HeLa cells were co-transfected with vectors expressing the indicated proteins. Cells were treated 48h post-transfection with or without 20µM MG132 for 6h, harvested and lysed. Proteins expression was analyzed by Western Blot. The left panel displays one representative experiment; the histogram shows the fold increase of sZIP expression (ratio over GFP) induced by MG132 with and without Vpr (7 independent experiments, p-value≈0.001). B. The DCAF1 binding-deficient Vpr mutant, VprQ65R, is less efficient than wt Vpr to induce sZIP degradation. HeLa cells were co-transfected with vectors expressing FLAG-sZIP, HA-tagged Vpr proteins as indicated and a GFP expression vector as an internal control (ratio 10:1). Cells were harvested 48h post-transfection, lysed and protein expression was analyzed by Western Blot (left panel, one representative experiment). The histogram shows the quantification of the ratio between the FLAG and GFP signals for 7 independent experiments. C. Silencing of DCAF1 impairs Vpr-induced sZIP degradation. HeLa cells were treated with either 50nM of control siRNA or with 50nM of siRNA directed against DCAF1. Cells were transfected 24h later with vectors expressing the indicated proteins. Cells were harvested 48h post-transfection, lysed and the proteins expression analyzed by Western Blot (left panel, one representative experiment). The histograms (right panel) display the ratios between FLAG and GFP signals.