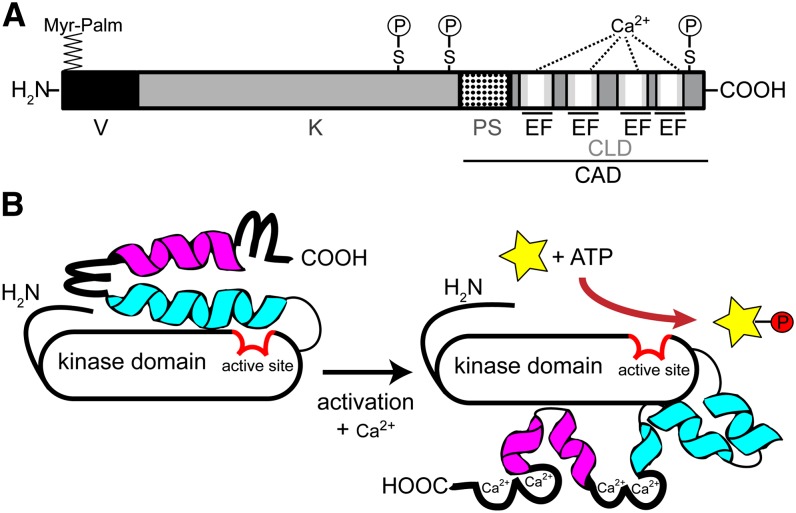
Figure 1.
Scheme of CDPK modular structure and activation. A, Domain structure of Ca2+-dependent protein kinase, drawn to scale for AtCPK28, consisting of 523 amino acids. V, N-terminal variable domain; K, kinase domain; PS, pseudosubstrate segment; CLD, calmodulin-like domain; EF, EF hand. The four white bars within the EF hand motifs represent the EF hand Ca2+-binding loop. The position of myristoylation and palmitoylation (Myr-Palm) and the in vivo autophosphorylation sites (P) at Ser (S) residues are depicted. B, Model for CDPK activation (modified after Wernimont et al., 2010). Calcium binding to the EF hand motifs (loop) induces a conformational change, in which helix 1 (blue) and helix 2 (magenta) break into segments, rotating the calmodulin-like domain around the kinase domain. This rotation increases the accessibility of the N-terminal variable domain as well as of the active site (red) in the kinase domain (black), allowing phosphorylation activity toward its substrate proteins (yellow star, P).