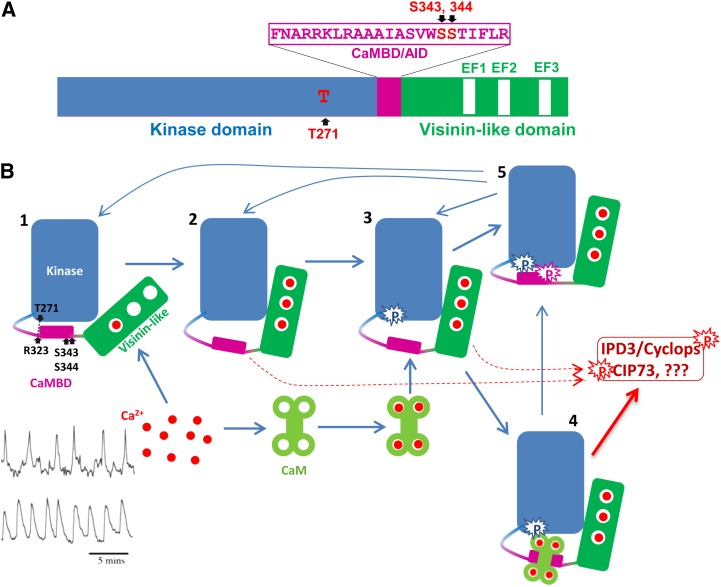
Figure 3.
A, Schematic diagram of MtCCaMK showing the domain structure and autophosphorylation sites. B, Activation of CCaMK by Ca2+ and Ca2+/CaM. State 1, CCaMK most likely with Ca2+ loaded in the first EF hand. CCaMK is inactive in this state. State 2, CCaMK loaded with Ca2+ in its three EF hands in the VLD. Because of the Ca2+-induced changes in the tertiary structure of CCaMK, the kinase is active and Thr-271 is ready for autophosphorylation. State 3, Thr-271 is autophosphorylated and CCaMK shows a dramatic increase in its affinity for CaM, a phenomenon called CaM trapping. State 4, Ca2+ and Ca2+/CaM-loaded CCaMK. The kinase is fully active and should be able to phosphorylate substrates as well as CCaMK itself. As long as the CCaMK is bound by Ca2+/CaM, it should remain active. State 5, Ser-343 and/or Ser-344 are phosphorylated, CCaMK can no longer interact with CaM, and its kinase activity is also turned off, whether the Ca2+ concentration is high enough to load in its EF hands. Mycorrhizal and Nod factor-induced Ca2+ spikings (bottom left corner: mycorrhizal is at top and Nod factor is at bottom) are adapted from Kosuta et al. (2008).