Abstract
The purpose of this study was to measure treatment outcomes in a group of six adults with chronic dysphagia following acquired brain injury, who each completed 24 sessions of tongue-pressure resistance training, over a total of 11–12 weeks. The treatment protocol emphasized both strength and accuracy. Biofeedback was provided using the Iowa Oral Performance Instrument. Amplitude accuracy targets were set between 20–90% of the patient's maximum isometric pressure capacity. Single subject methods were used to track changes in tongue strength (maximum isometric pressures), with functional swallowing outcomes measured using blinded ratings of a standard pre- and post-treatment videofluoroscopy protocol. Improvements were seen in post-treatment measures of tongue pressure and penetration–aspiration. No improvements were seen in pharyngeal residues, indeed worsening residue was seen in some patients.
Keywords: Deglutition, swallowing, rehabilitation, tongue, manometry, treatment, intervention, speech-language pathology.
Introduction
Dysphagia (swallowing impairment) is a serious condition of increasing burden and importance in our ageing society (Gonzalez-Fernandez & Daniels, 2008; Humbert & Robbins, 2008; Kikawada, Iwamoto, & Takasaki, 2005; Pikus, Levine, Yang, Rubesin, Katzka, & Laufer, 2003). It is estimated that 37–78% of the 750,000 Americans who suffer a stroke each year will experience dysphagia (Martino, Foley, Bhogal, Diamant, Speechley, & Teasell, 2005; www.stroke.org) and that 43–54% of these individuals will experience aspiration (entry of foreign material into the airway) (Daniels, Brailey, Priestly, Herrington, Weisberg, & Foundas, 1998; Horner, Massey, Riski, Lathrop, & Chase, 1988). Stroke patients who aspirate face 11.56-times the risk of developing pneumonia, compared to those without dysphagia (Martino et al., 2005). In the acquired brain injury (ABI) population, dysphagia has a similar reported incidence of 41–61% (Halper, Cherney, Cichowski & Zhang, 1999; Winstein, 1983), with videofluoroscopically-confirmed aspiration incidence reported at 38% (Lazarus & Logemann, 1987).
Impaired tongue function is a prominent component in neurogenic dysphagia; the ability to generate tongue-pressure plays a primary role in controlling the flow of liquids through the mouth and pharynx (Pouderoux & Kahrilas, 1995). The tongue is also responsible for initiating the driving forces that propel liquids and foods through the oropharynx into the esophagus (Kahrilas, Lin, Logemann, Ergun, & Facchini, 1993). When tongue-pressure generation is impaired, liquids may spill into the pharynx before the airway is protected, or bolus clearance may be impaired, leaving residue in the pharynx. In both of these situations, there is a heightened risk of aspiration (Marik & Kaplan, 2003; Martino et al., 2005; Pikus et al., 2003). Studies have shown that an emphasis on tongue–palate pressures during the performance of effortful swallows enhances the generation of increased pharyngeal pressures and speeds up propagation of the pharyngeal pressure wave (Huckabee & Steele, 2006; Steele & Huckabee, 2007). These findings suggest that tongue–palate pressure generation plays a pivotal role in establishing the overall strength of a swallow, and establishes a theoretical basis for using tongue–palate pressure exercises as an intervention to target pharyngeal bolus clearance.
The most common intervention for managing aspiration risk in oropharyngeal dysphagia is to prescribe thickened liquids and texture-modified foods (Garcia, Chambers, & Molander, 2005). Unfortunately, texture-modifications are reported to be unpalatable and patients are prone to dehydration and inadequate nutritional intake (Colodny, 2005; Robbins, Gensler, Hind, Logemann, Lindblad, & Brandt, 2008; Sharpe, Ward, Cichero, Sopade, & Halley, 2007). Consequently, it is important that dysphagia researchers explore treatments that facilitate the return of functional swallowing without requiring texture modification. In this study, we describe treatment outcomes for a tongue-pressure resistance training intervention.
Over the past decade, the field of dysphagia intervention research has turned to using principles of exercise physiology in treatment design. Robbins developed an 8-week tongue-pressure resistance training protocol, which she applied in healthy seniors and individuals with dysphagia post-stroke (Robbins, Gangnon, Theis, Kays, Hewitt, & Hind, 2005; Robbins, Kays, Gangnon, Hind, Hewitt, & Gentry, 2007). The Robbins protocol employs elements of exercise load, intensity, and treatment duration, intended to support physiological changes in tongue muscle strength and capacity. Patients practice repeated tongue-pressure tasks, with targets set between 60–80% of their maximum capacity, on a schedule requiring 60–90 task repetitions on 3 non-consecutive days of the week. Performance-contingent biofeedback is provided using a hand-held manometer. Using this protocol, Robbins et al. effectively demonstrated that tongue-pressure resistance training builds tongue strength. In a group of 10 patients with stroke-related dysphagia of up to 3 months duration, anterior maximum isometric tongue pressures (MIPs), i.e., the highest pressure achieved during a maximum effort task while compressing a palatal pressure sensor with the tongue, improved by 44% vs baseline, while posterior MIPs increased by 83% (Robbins et al., 2007). Unfortunately, there was less convincing evidence that functional swallowing improvements accompanied these strength changes. Post-treatment videofluoroscopy showed a significant groupwise improvement in aspiration, but there were no systematic changes in pharyngeal residues. Quite why this pattern of change was observed remains unclear, but the possibility that spontaneous recovery played some role in these outcomes cannot be dismissed.
Swallowing is recognized to be a sub-maximal pressure task (Nicosia, Hind, Roecker, Carnes, Doyle, & Dengel, 2000). Although a decline in MIPs is seen with ageing in healthy individuals, this is not seen in the context of swallowing (Nicosia et al., 2000; Youmans, Youmans, & Stierwalt, 2009). Given this situation, we speculated that the task of trying to hit variable amplitude targets precisely, without over-shoot or under-shoot, might involve skills similar to those involved in varying tongue pressures for liquid bolus control in swallowing. We designed a modification to the Robbins protocol, emphasizing amplitude accuracy targets in the 20–90% of MIP range in addition to strength targets (Tongue-Pressure Strength and Accuracy Training, or TPSAT). We hoped that the modification of including variable target practice would still facilitate strength gains, while achieving improved functional outcomes for safe swallowing and residue clearance. This manuscript describes a preliminary prospective case series of six patients with dysphagia secondary to acquired brain injury who completed this protocol. We were interested in determining whether the TPSAT protocol would yield improvements in three areas: (1) tongue strength for MIPs and saliva swallows; (2) swallowing safety; and (3) swallowing efficiency.
Method
The study received human subjects approval from the hospital's institutional review board. We enrolled six consenting participants with dysphagia secondary to acquired brain injury following a motor vehicle accident, with injuries sustained either as a driver, passenger or pedestrian. Table I provides summary information about these individuals, including the available medical history information regarding the nature of their brain injuries. All participants were at least 5 months post-onset and had no pre-accident history of dysphagia. All participants underwent a standard baseline videofluoroscopy protocol to confirm eligibility to participate. The inclusion criteria for the study were as follows:
Table I.
Participant demographics and baseline videofluoroscopy impairment profiles.
| Participant | Gender | Age | Description of injury | Time post-injury at start of protocol | Diet at start of protocol |
|---|---|---|---|---|---|
| 1 | F | 45 | Intracranial haemorrhage. Fractures: C5–C6. | 6 months | Gastrostomy feeding, nil by mouth. |
| 2 | M | 32 | Intraventricular haemorrhage, diffuse axonal injury, frontal lobe white matter density changes. Partial left lung lobectomy following major aspiration event 24 months prior to enrolment. | 42 months | Gastrostomy feeding, nil by mouth. |
| 3 | M | 47 | Closed head injury, epidural haematoma. | 6 months | Oral minced diet with honey-thick liquids, no fibrous textures. |
| 4 | M | 54 | Closed head injury, subarachnoid haemorrhage. Fractures: ribs, C6–7, T1–3. | 18 months | Gastrostomy feeding. Therapeutic trials of water, soft solids. |
| 5 | M | 32 | Closed head injury and multiple musculoskeletal injuries (not specified). | 24 months | Oral minced diet with nectar-thick liquids. |
| 6 | F | 44 | Diffuse axonal injury, bilateral frontal lobe contusions, subarachnoid and left occipital horn haemorrhage. Left flail chest, pneumo-mediastinum, pneumothorax. Fractures: ribs, L1, L2, L5, pelvis. Lacerated liver. GCS 7. | 5 months | Gastrostomy feeding, nil by mouth. |
(1) impaired swallowing safety, i.e., scores ≥ 3 on the 8-point Penetration-Aspiration Scale (Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996) with thin liquids; and
(2) post-swallow residues in the valleculae or pyriform sinuses with either thin and/or spoon-thick liquids measured using a 4-point ordinal scale described by Eisenhuber, Schima, Schober, Pokieser, Stadler, and Scharitzer (2002): 0 = none, 1 = thin coating, 2 = 25–50% full, 3 ≥ 50% full.
Table II provides an overview of the videofluoroscopy profiles of each participant at baseline, reflecting the worst scores received by each participant for each bolus consistency on the three parameters of interest.
Table II.
Baseline swallowing status from pre-treatment videofluoroscopy.
| Participant | Baseline swallowing status (videofluoroscopy) |
|||
|---|---|---|---|---|
| Stimulus | Penetration–aspiration scale scorea | Vallecular residue scoreb | Pyriform sinus residue scoreb | |
| 1 | Thin | 8 | 2 | 2 |
| Spoon-Thick | 8 | 2 | 2 | |
| 2 | Thin | 8 | 0 | 2 |
| Spoon-Thick | 5 | 0 | 2 | |
| 3 | Thin | 8 | 1 | 0 |
| Spoon-Thick | 5 | 2 | 1 | |
| 4 | Thin | 7 | 2 | 1 |
| Spoon-Thick | 1 | 2 | 2 | |
| 5 | Thin | 8 | 1 | 0 |
| Spoon-Thick | 1 | 1 | 0 | |
| 6 | Thin | 8 | 2 | 1 |
| Spoon-Thick | 8 | 3 | 1 | |
aPenetration–aspiration was rated using the 8-point Penetration Aspiration Scale (Rosenbek et al., 1996) in which a score of 1 reflects normal swallowing safety, a score of 2 indicates high penetration of the supraglottic space with subsequent ejection, scores of 3–5 indicate laryngeal penetration, and 6–8 indicate aspiration of material below the true vocal folds.
bResidues in the valleculae and pyriform sinuses were rated using a 4-point ordinal scale described by Eisenhuber et al. (2002) in which 0 = no residue, 1 = thin coating, 2 = 25–50% full, and 3 ≥ 50% full.
All participants received 24 sessions of treatment, scheduled twice weekly (with a third session occasionally scheduled within the same week), and spanning a total of 11–12 weeks. This schedule was dictated by practical constraints due to the fact that several of the patients were commuting as outpatients to the hospital to participate. Treatment was provided by a licensed research speech-language pathologist.
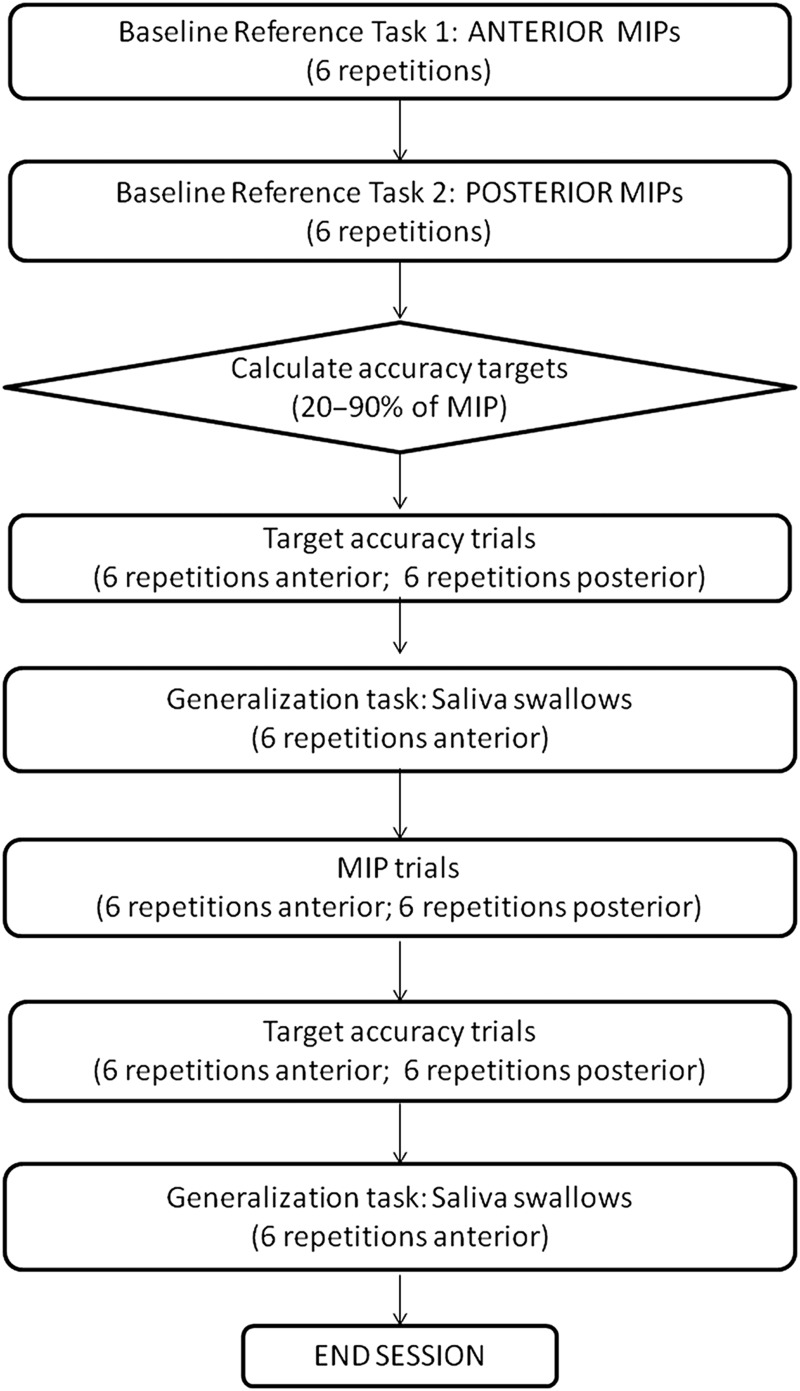
Figure 1 outlines the design of a treatment session, which involved a total of 60 tongue-pressure tasks, arranged in blocks of six, with individual task repetitions separated by a minimum 10-second rest period. All tasks were performed using the Iowa Oral Performance Instrument (IOPI), a hand-held manometer connected to a 2.7 millilitre air-filled pressure bulb that was positioned between the tongue and hard palate in either an anterior or posterior oral position. The first two task-blocks in the session were MIPs (anterior and posterior), from which 20% and 90% of MIP values were calculated. For the variable accuracy tasks that followed, the clinician randomly selected variable target values between these 20% and 90% boundaries and instructed the participant to try to hit each target pressure amplitude as accurately as possible. Feedback was provided to the participant after each task repetition, by telling them the pressure amplitude shown on the IOPI. Equal time was spent on strength tasks (MIPs) and target accuracy tasks in each session. Additionally, a generalization task of saliva swallows was included with the IOPI bulb placed in the anterior position. These swallows were cued saliva swallows with as much time allowed as required between task repetitions. The bulb was removed between each swallow and repositioned, thereby allowing a brief rest and allowing the patient to indicate when they were ready to proceed.
Figure 1.
Flow-chart of a tongue-pressure strength and accuracy training session.
Single-subject descriptive methods were used to report treatment progress for the tongue pressure parameters, which were probed at each treatment session. A control chart method with effect-size banding (Nourbakhsh & Ottenbacher, 1994) was used to confirm the presence or absence of change, as follows:
(1) Measures of baseline tongue pressure range, by task, were computed using mean and standard deviation values for each participant for all MIP tasks across the first three treatment sessions (n = 36 in the anterior and 36 in the posterior sensor positions). This range constituted the baseline reference range to which subsequent performance was compared.
(2) Means and standard deviations for tongue-pressure amplitudes were calculated separately by task from the IOPI data collected during each session: 12 anterior MIPs, 12 posterior MIPs, and 12 non-effortful saliva swallows. These values were plotted on control charts to document change in tongue pressure parameters over time (see Figure 2).
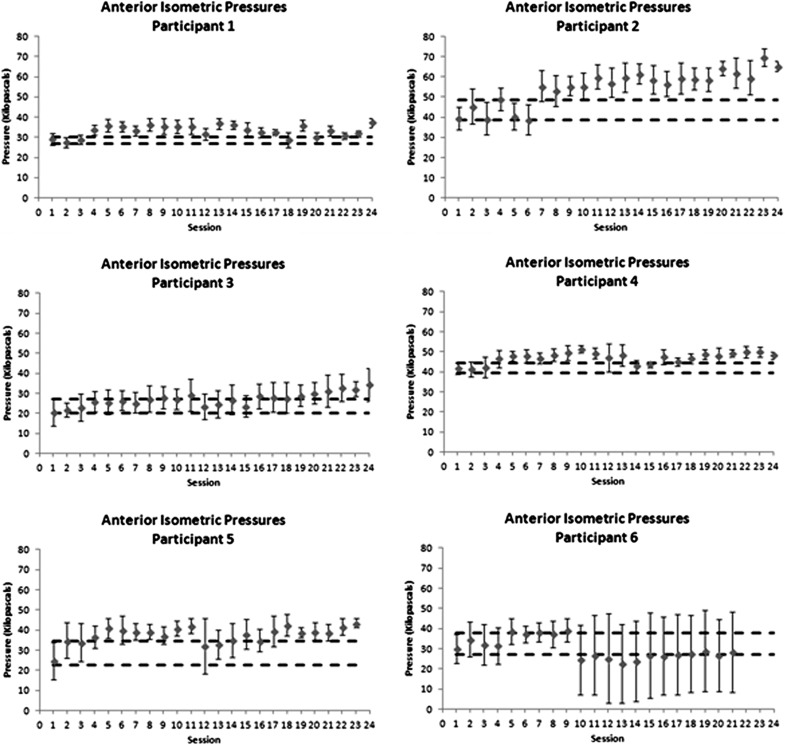
Figure 2.
Control charts showing progress in anterior maximum isometric tongue–palate pressures over the course of therapy. Dashed horizontal lines indicate a moderate effect size band around baseline performance, used as a threshold to determine whether there was evidence of change in the form of at least three consecutive data points exceeding the effect size band.
(3) An effect size band of Cohen's d = ± 0.6, i.e., a strong effect (Kotrlik & Williams, 2003), was established around these baseline mean values and plotted using dashed lines above and below the baseline reference range on the control charts.
(4) An a priori criterion for concluding that change in MIP values had occurred was established as evidence of three or more consecutive data points falling outside the effect-size band boundaries.
Measures of functional swallowing outcome were derived from post-treatment videofluoroscopies (compared to baseline), performed according to a standard protocol using a 40% weight/volume thin liquid solution of Polibar (Bracco Imaging S.p.A., Princeton, New Jersey) and water, and a spoon-thick liquid prepared using EZ-HD barium power (Bracco), mixed in 40% weight/volume ratio with applesauce. The standard protocol included three repetitions of each of these stimuli, all delivered in 5-ml volumes using a spoon and swallowed using a command swallow in head neutral position without any compensations. Additional tasks were included at clinician discretion, and included other consistencies (nectar-thick, honey-thick, or solid consistencies) and the exploration of compensatory maneouvres, but these were not considered for the purposes of measuring treatment outcome in the research study.
The videofluoroscopic measures of interest were the 8-point Penetration-Aspiration Scale (Rosenbek et al., 1996) and 4-point ordinal ratings of residue in the valleculae and pyriform sinuses (Eisenhuber et al., 2002). Ratings were made by two experienced speech-language pathologists, who had initially been trained to consensus in the scoring of these features using a training set of three videofluoroscopy recordings from patients not involved in this study. The videofluoroscopy recordings of the head-neutral, non-compensated thin liquid and spoon-thick liquid swallows were spliced into bolus-specific clips. These were arranged in random order and divided into two rating sets with 20% overlap between raters for the purposes of measuring inter-rater agreement. Using this partial reliability dataset, intra-class coefficients (ICC) for inter-rater agreement were strong for all measures: penetration-aspiration ICC = .937, 95% CI = .86–.97; vallecular residue ICC = .94, 95% CI = .86–.97; pyriform sinus residue, ICC = .917, 95% CI = .81–.96). Based on this strong performance for the reliability dataset, it was decided that duplicate ratings of the entire dataset across both raters was not necessary. Disagreements for individual ratings within the reliability dataset were resolved by consensus. For the purposes of the research study, it was decided a priori that the worst scores seen across all swallows for a given bolus consistency would be used to represent a patient's functional swallowing status at each time-point (pre-, post-treatment).
Results
Table III summarizes mean and standard deviation values for the three tongue-pressure parameters of interest, calculated across the baseline timeframe spanning the first three treatment sessions for each participant. Figures 2–4 provide a graphic summary of the progression of tongue pressure measures for each participant across the course of the study.
Table III.
Baseline measures of tongue strength (means and standard deviations) calculated over the first three treatment sessions, with 12 repetitions of each task per session.
|
Participant |
Baseline MIP (KPa) |
||
|---|---|---|---|
| Anterior | Posterior | ||
| 1 | Mean | 28.4 | 26.6 |
| SD | 2.7 | 3.6 | |
| 2 | Mean | 43.5 | 26.8 |
| SD | 7.5 | 5.2 | |
| 3 | Mean | 23.7 | 17.2 |
| SD | 5.7 | 5.1 | |
| 4 | Mean | 41.9 | 21.4 |
| SD | 3.8 | 2.6 | |
| 5 | Mean | 28.4 | 23.3 |
| SD | 9.2 | 7.6 | |
| 6 | Mean | 32.4 | 20.3 |
| SD | 8.6 | 9.1 | |
Figure 3.
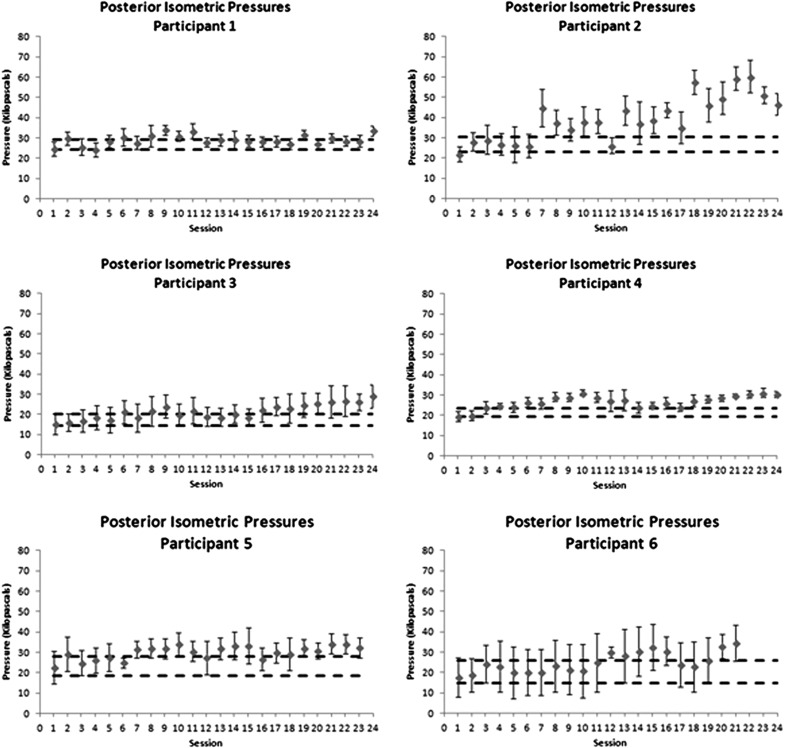
Control charts showing progress in posterior maximum isometric tongue–palate pressures over the course of therapy. Dashed horizontal lines indicate a moderate effect size band around baseline performance, used as a threshold to determine whether there was evidence of change in the form of at least three consecutive data points exceeding the effect size band.
Figure 4.
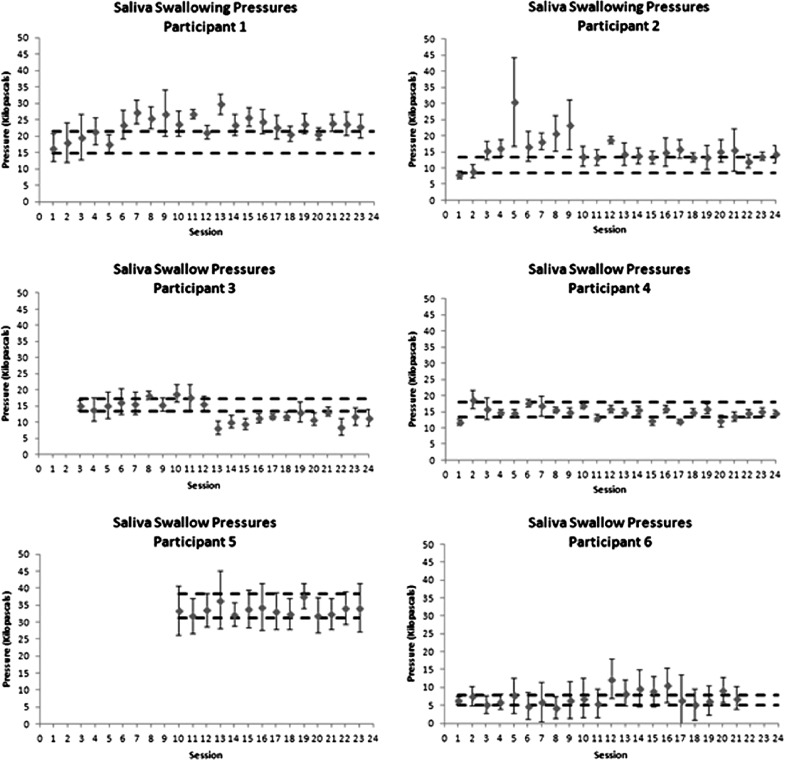
Control charts showing progress in saliva swallowing pressures over the course of therapy. Dashed horizontal lines indicate a moderate effect size band around baseline performance, used as a threshold to determine whether there was evidence of change in the form of at least three consecutive data points exceeding the effect size band.
The charts showing anterior (Figure 2) and posterior maximum isometric pressures (Figure 3) are graphed using a uniform y-axis range of 0–80 kPa. For the reader's reference, it may be useful to note that previous studies have suggested that the lower boundary for normal anterior isometric pressures in elderly men falls at ˜ 40 kPa (Nicosia et al., 2000). In the case of this study, it can be appreciated that all participants except participants 2 and 4 began treatment with anterior MIPs below this threshold. Over the course of treatment, increased anterior MIPs (sustained above the Cohen's d = 0.6 effect size boundary for at least three consecutive sessions) can be seen for participants 1, 2, 3, 4, and 5. Participants 1 and 4 failed to maintain these gains across the entire course of treatment. Participant 6 showed an unusual pattern with respect to anterior MIPs, which increased over the first 10 sessions, but then declined below the d = .6 effect size boundary for the second half of treatment, and displayed much greater variability as seen in the standard deviation error bars shown on the chart. This suggests that some change in the strategy used to perform MIPs occurred after the first 10 sessions, rendering the initial baseline measures inappropriate as a comparison for the second half of treatment.
At baseline, posterior MIPs were below 40 kPa in all six participants (Figure 3). Increases in posterior tongue–palate pressures, beyond the d = .6 effect size boundary, were seen over at least three consecutive treatment sessions for all six participants; however, sustained gains were not seen for participant 1.
Saliva swallowing pressures are plotted in Figure 4 using a uniform y-axis range of 0–50 kPa, based on evidence that saliva swallow pressures typically fall below 50% of MIPs (Nicosia et al., 2000). For the six participants in this study, saliva swallow pressures were at or below 20 kPa in all cases at baseline. The only exception to this was seen in participant 5, who was unable to produce volitional saliva swallows at baseline. When these emerged at around session 10, his saliva swallow pressures were in the range of 30–40 kPa and similar in amplitude to his MIPs. Increases in saliva swallowing pressures, beyond the effect size boundary over at least three consecutive sessions were seen for participants 1, 2, and 6, but gains were not clearly sustained for participants 2 and 6. Participants 3, 4, and 5 failed to demonstrate any notable changes in saliva swallowing pressures.
Table IV summarizes treatment outcomes in terms of the functional swallowing measures derived from the videofluoroscopies in this study. When improvement was seen compared to baseline, this is indicated by a black font on a white background; deterioration is indicated using a white font on a black background, while an absence of change is shown using a black font on a grey background.
Table IV.
Post-treatment swallowing outcomes from videofluoroscopy, by participant.
| Participant | Stimulus | Penetration–aspiration scale scorea | Vallecular residue scoreb | Pyriform sinus residue scoreb |
|---|---|---|---|---|
| 1 | Thin | 5 (improved) | 2 (unchanged) | 3 (worse) |
| Spoon-Thick | 5 (improved) | 3 (worse) | 3 (worse) | |
| 2 | Thin | 1 (improved) | 2 (worse) | 1 (improved) |
| Spoon-Thick | 1 (improved) | 2 (worse) | 2 (unchanged) | |
| 3 | Thin | 8 (unchanged) | 2 (worse) | 0 (unchanged) |
| Spoon-Thick | 3 (improved) | 2 (unchanged) | 0 (improved) | |
| 4 | Thin | 2 (improved) | 2 (unchanged) | 2 (worse) |
| Spoon-Thick | 2 (worse) | 3 (worse) | 3 (worse) | |
| 5 | Thin | 4 (improved) | 2 (worse) | 1 (worse) |
| Spoon-Thick | 8 (worse) | 2 (worse) | 0 (unchanged) | |
| 6 | Thin | 4 (improved) | 2 (unchanged) | 1 (unchanged) |
| Spoon-Thick | 1 (improved) | 3 (unchanged) | 0 (improved) |
Roman text in the table reflect a functional swallowing improvement in comparison to the pre-treatment videofluoroscopy, while italics reflect no noticeable change and underlined text indicates deterioration in function for that bolus/parameter combination, relative to the baseline evaluation.
aPenetration–aspiration was rated using the 8-point Penetration Aspiration Scale (Rosenbek et al., 1996) in which a score of 1 reflects normal swallowing safety, a score of 2 indicates high penetration of the supraglottic space with subsequent ejection, scores of 3–5 indicate laryngeal penetration, and 6–8 indicate aspiration of material below the true vocal folds.
bResidues in the valleculae and pyriform sinuses were rated using a 4-point ordinal scale described by Eisenhuber et al. (2002) in which 0 = no residue, 1 = thin coating, 2 = 25–50% full, and 3 ≥ 50% full.
Improved swallowing safety for thin liquids was seen post-treatment for participants 1, 2, 4, 5, and 6, but remained unchanged for participant 3. Impaired swallowing safety for spoon-thick liquids was a concern at baseline in participants 1, 2, 3, and 6; post-treatment improvement was seen in all four of these participants. However, it should also be noted that two participants who had shown baseline safety (scores of 1) with spoon-thick liquids showed new evidence of impaired swallowing safety with this consistency on their post-treatment videofluoroscopies; participant 4 showed high penetration (score of 2), while participant 5 showed silent aspiration (score of 8).
Vallecular residue with thin liquids was considered problematic at baseline (i.e., scores of 2 or higher) in participants 1, 4, and 6, but remained unchanged post-treatment in all three cases. In participant 3, baseline evidence of mild vallecular residue (i.e., a score of 1) with thin liquids actually worsened to moderate residue post-treatment (i.e., progression from a score of 1 to 2). With spoon-thick liquids, vallecular residue was a concern at baseline for participants 1, 3, 4, and 6. Scores remained unchanged post-treatment for participants 3 and 6, but deteriorated by one grade of severity on the Eisenhuber rating scale in both participants 1 and 4.
Pyriform sinus residue scores of 2 or higher for thin liquids were seen at baseline in participants 1 and 2. Participant 2 showed post-treatment improvement in this measure, while participant 1 showed deterioration by one grade of severity to a post-treatment score of 3. Additionally, post-treatment measures for participant 4 revealed moderate pyriform residue for thin liquids, when only mild residue had been seen at baseline, a worsening from a score of 1–2. Pyriform sinus residue scores of 2 or higher for spoon-thick liquids at baseline were seen in participants 1, 2, and 4. Post-treatment, this feature remained unchanged in participant 2, while participants 1 and 4 showed a worsening to scores of 3.
Overall, these results show improvement in swallowing safety with thin liquids for the majority, and improved safety with spoon-thick liquids in about half of these patients. Changes in post-swallow vallecular and pyriform sinus residues are much less apparent, and there is no trend of improvement in this group of patients. Indeed, the data suggest that there was worsening of bolus clearance in many of these patients at the end of therapy. These results were seen in the context of improved tongue strength for MIPs.
Discussion
Our results show that all six participants in this study achieved increases in either anterior and/or posterior tongue strength on MIPs post-treatment. These results are interesting for several reasons. First of all, improvements were achieved in individuals who had dysphagia of long-standing (minimum 5 months). Even the participants with dysphagia of longer than 18 months chronicity demonstrated convincing improvements in both anterior and posterior tongue strength, thereby speaking to the ongoing plasticity of the tongue and its potential to respond to exercise training in this population. Secondly, the observed improvements in strength are interesting given that the focus of our treatment was equally distributed between maximum isometric tasks and accuracy tasks requiring the participant to hit sub-maximal pressure targets as precisely as possible. This finding is reminiscent of findings in the limb rehabilitation literature, where it has been shown that strength gains can arise from skill-focused exercise (Monfils & Teskey, 2004; Winstein, Rose, Tan, Lewthwaite, Chui, & Azen, 2004). Taken together with the previous results reported by Robbins et al. (2007), these data confirm that tongue-pressure resistance exercises, practiced 2–3-times weekly, with amplitude targets tailored to the patient's maximum pressure capacity, are an effective method for increasing tongue strength in individuals with neurogenic dysphagia.
Unfortunately, the types and degree of functional swallowing changes that accompanied these improvements in tongue strength are less clear. In this study, the majority of participants showed a functional improvement in aspiration. Five of the six patients displaying aspiration on thin liquids at baseline showed resolution of this impairment at the post-treatment videofluoroscopy. This finding concurs with the results reported by Robbins et al. (2007), despite the fact that our participants had dysphagia of longer chronicity than the stroke patients in her study. Nevertheless, it must be acknowledged that spontaneous recovery may have contributed to the improvements seen in our participants and it remains unclear to what extent the improvement in aspiration can be attributed to tongue-pressure resistance training.
In contrast, in the current data set, we did not observe any positive trends with respect to improved bolus clearance and reduced residues in the valleculae and pyriform sinuses. In fact, the videofluoroscopy results appear to show deterioration in bolus clearance function in five of our six participants, and there is no apparent pattern to these results with respect to dysphagia chronicity or severity, in terms of pre-treatment diet tolerance. These results are disappointing and raise challenging questions regarding the possibility that there was some negative impact with this treatment protocol. On the one hand, the lack of improvement in residue clearance adds support to the speculation that spontaneous recovery may have contributed to some extent to the observed improvements in swallowing safety. On the other hand, it must be remembered that the observed results reflect each participant's worst score across a series of three thin liquid and three spoon-thick liquid boluses at each assessment. The apparent worsening may simply reflect variability in swallowing function within these participants within a limited videofluoroscopy protocol, given the long-standing nature of their dysphagia. We feel that it would be an over-interpretation of these data to conclude that these individuals showed functional deterioration in their swallowing, even in the presence of at least one residue score at the post-treatment videofluoroscopy that was more severe than that observed at baseline. However, the fact that residues appeared to be resistant to change in the presence of increased tongue pressures does raise questions about one of the underlying assumptions of this study, namely that tongue driving force contributes to effective bolus clearance from the pharynx (Dejaeger, Pelemans, Ponette, & Joosten, 1997). Our data suggest that the tongue-pressure resistance exercises practiced in this study did not yield generalized improvements in pharyngeal swallowing strength, although we had hoped to see such generalization based on evidence regarding the impact of effortful swallows involving heightened tongue pressure generation on pharyngeal pressure generation and propagation (Huckabee & Steele, 2006; Steele & Huckabee, 2007). Fortunately, perhaps, we do not appear to be alone in our failure to achieve positive results with respect to bolus clearance through exercise-based approaches to swallowing therapy. Robbins et al. (2007) similarly failed to find convincing evidence of improvements in bolus clearance with their tongue pressure training protocol, even given shorter chronicity of dysphagia and a clearer emphasis on building tongue strength in their protocol. Similarly, prior studies of the Shaker exercise and neuromuscular electrical stimulation have also failed to show convincing evidence of physiological improvements in bolus clearance in patients with neurogenic dysphagia (Bülow, Speyer, Baijens, Woisard, & Ekberg, 2008; Logemann, Rademaker, Pauloski, Kelly, Stangl-McBreen, & Antinoja, 2009; Robbins et al., 2007).
Overall, the less-than-satisfactory findings in these six participants suggest a need to better understand the role that tongue pressure generation plays in relation to other functional aspects of swallowing physiology, such as pharyngeal bolus clearance. For example, there may be aspects of the oral phase of swallowing that are more sensitive to the influence of tongue pressure amplitudes than the parameters measured in our study. Indeed, our original plan had been to include measures related to oral control of the bolus in this study, but we determined that the literature lacked evidence regarding valid quantitative measures of bolus control that would lend themselves to reflecting the possible influence of treatment. Stage transition duration, for example, is a quantitative measure that captures the interval following arrival of the bolus in the pharynx prior to the onset of hyolaryngeal motion associated with the pharyngeal phase of the swallow (Lof & Robbins, 1990). While stage transition duration would definitely be prolonged in a situation of impaired oral bolus control resulting in premature spillage of material into the pharynx, there are no clear guidelines for dissociating this type of impairment from delayed pharyngeal swallow response. Furthermore, research has shown that there can be considerable variation, even amongst healthy individuals in the position of the head of the bolus at swallow onset (Martin-Harris, Brodsky, Michel, Lee, & Walters, 2007), making the objective delineation of impairment in liquid bolus control quite challenging.
In this study, we report data from six participants in whom bolus clearance measures did not improve, despite measurable improvements in tongue strength. Several comments may be appropriate in response to this observation. First, it must be recognized that neither this treatment protocol, nor the one used by Robbins et al. (2007), included any swallowing tasks (saliva or other material) in which there was a specific instruction to use heightened tongue pressure while executing the swallow. It may be that this sort of specific emphasis on generating swallows involving higher pressure amplitudes would be necessary to support generalization and that tongue-pressure tasks, which are not immediately followed by a swallow, lack specificity for improving swallowing function (Ferguson & Rice, 2001). Another possibility is that there are aspects of tongue pressure generation in swallowing, other than the amplitudes that are generated, that are relevant for effective bolus propulsion and clearance. Recent work has shown that healthy individuals modulate both the amplitude and time-scale of tongue-pressures when swallowing liquids of differing consistency (Steele, Bailey, & Molfenter, 2010). To date, tongue-pressure resistance training has ignored temporal parameters. It may be that treatment protocols need to incorporate this aspect by emphasizing tasks that mimic healthy swallows in their amplitude-time profile in order to achieve improved functional outcomes with respect to bolus control (Steele, Bailey, Molfenter, Yeates, & Grace-Martin, 2010).
Study limitations
A sample of only six participants is clearly under-powered to detect groupwise changes in swallowing. For this reason, we adopted single subject methods for data analysis and reporting. As previously acknowledged, although participants were at least 5 months post-onset, it is still possible that spontaneous recovery contributed to some of the changes seen. Additionally, our decision to take a conservative approach by using the worst scores observed to represent a patient's function may have contributed to the lack of apparent change. Our experience using ordinal rating scales to capture residue severity also suggests that such scales lack resolution for detecting change in statistical analyses. Perceptual judgements of the amount of residue occupying the valleculae or pyriform sinuses, categorized in quarterly increments, lack resolution and validation and are not sensitive to subtle changes that may or may not be present with real functional change.
Finally, it may be that modifications to the schedule and intensity of treatment used in this study might lead to different treatment outcomes. Participants in this study attended sessions twice weekly, as a rule, over the course of 11–12 weeks. This schedule was found to be feasible, even for patients who were commuting to treatment as outpatients, and could be scheduled in conjunction with any other medical appointments that were required during participation in the study. Whether treatment intensification over a different time frame (either shorter or longer) would lead to different outcomes is a question for future research.
Conclusions
In conclusion, this study shows that a tongue-pressure resistance training protocol emphasizing strength and accuracy targets results in improved tongue strength measures, both in swallowing and in isometric tasks, and in improved penetration–aspiration scores. However, post-swallow pharyngeal residues did not improve as a result of treatment and, in fact, showed apparent deterioration in some cases.
Acknowledgements
The authors gratefully acknowledge assistance provided by Melanie Moore, Ashley Waito, and Tiffany Fei with data collection and processing. The authors acknowledge the support of the Toronto Rehabilitation Institute, which receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-term Care in Ontario. The views expressed do not necessarily reflect those of the Ministry.
Declaration of interest: The authors report no conflicts of interest.
This material was based on work supported by operating and career award grants from the Canadian Institutes of Health Research (grants 69521, 82668, 84534, and 83888). Additional funding support was provided by the Toronto Rehabilitation Institute and an Ontario Ministry of Research and Innovation Early Researcher Award to the first author.
References
- 1.Bülow M., Speyer R., Baijens L., Woisard V., Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. (2008);23:302–309. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 2.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. American Journal of Speech-Language Pathology. (2005);14:61–70. doi: 10.1044/1058-0360(2005/008). [DOI] [PubMed] [Google Scholar]
- 3.Daniels S. K., Brailey K., Priestly D. H., Herrington L. E., Weisberg L. A., Foundas A. L. Aspiration in patients with acute stroke. Archives of Physical Medicine and Rehabilitation. (1998);79:14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- 4.Dejaeger E., Pelemans W., Ponette E., Joosten E. Mechanisms involved in postdeglutition retention in the elderly. Dysphagia. (1997);12:63–67. doi: 10.1007/PL00009520. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhuber E., Schima W., Schober E., Pokieser P., Stadler A., Scharitzer M. Videofluoroscopic assessment of patients with dysphagia: Pharyngeal retention is a predictive factor for aspiration. American Journal of Roentgenology. (2002);178:393–398. doi: 10.2214/ajr.178.2.1780393. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson M. C., Rice S. The effect of contextual relevance on motor skill transfer. American Journal of Occupational Therapy. (2001);55:558–565. doi: 10.5014/ajot.55.5.558. [DOI] [PubMed] [Google Scholar]
- 7.Garcia J. M., Chambers IV E., (the 4th), Molander M. Thickened liquids: Practice patterns of speech-language pathologists. American Journal of Speech-Language Pathology. (2005);14:4–13. doi: 10.1044/1058-0360(2005/003). [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Fernandez M., Daniels S. K. Dysphagia in stroke and neurologic disease. Physical Medicine and Rehabilitation Clinics of North America. (2008);19:867–888. doi: 10.1016/j.pmr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Halper A. S., Cherney L. R., Cichowski K., Zhang M. Dysphagia after head trauma: The effect of cognitive-communicative impairments on functional outcomes. Journal of Head Trauma and Rehabilitation. (1999);14:486–496. doi: 10.1097/00001199-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Horner J., Massey E. W., Riski J. E., Lathrop D. L., Chase K. N. Aspiration following stroke: Clinical correlates and outcome. Neurology. (1988);38:1359–1362. doi: 10.1212/wnl.38.9.1359. [DOI] [PubMed] [Google Scholar]
- 11.Huckabee M. L., Steele C. M. An analysis of lingual contribution to submental surface electromyograph measures and pharyngeal pressure during effortful swallow. Archives of Physical Medicine and Rehabilitation. (2006);87:1067–1072. doi: 10.1016/j.apmr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Humbert I. A., Robbins J. Dysphagia in the elderly. Physical Medicine and Rehabilitation Clinics of North America. (2008);19:853–866. doi: 10.1016/j.pmr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahrilas P. J., Lin S., Logemann J. A., Ergun G. A., Facchini F. Deglutitive tongue action: Volume accommodation and bolus propulsion. Gastroenterology. (1993);104:152–162. doi: 10.1016/0016-5085(93)90847-6. [DOI] [PubMed] [Google Scholar]
- 14.Kikawada M., Iwamoto T., Takasaki M. Aspiration and infection in the elderly: Epidemiology, diagnosis and management. Drugs and Aging. (2005);22:115–130. doi: 10.2165/00002512-200522020-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kotrlik J. W., Williams H. A. The incorporation of effect size in informaton technology, learning, and performance research. Information Technology, Learning, and Performance Journal. (2003);21:1–7. [Google Scholar]
- 16.Lazarus C., Logemann J. A. Swallowing disorders in closed head trauma patients. Archives of Physical Medicine and Rehabilitation. (1987);68:79–84. [PubMed] [Google Scholar]
- 17.Lof G. L., Robbins J. Test-retest variability in normal swallowing. Dysphagia. (1990);4:236–242. doi: 10.1007/BF02407271. [DOI] [PubMed] [Google Scholar]
- 18.Logemann J. A., Rademaker A., Pauloski B. R., Kelly A., Stangl-McBreen C., Antinoja J. A randomized study comparing the Shaker exercise with traditional therapy: A preliminary study. Dysphagia. (2009);24:403–411. doi: 10.1007/s00455-009-9217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marik P. E., Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. (2003);124:328–336. doi: 10.1378/chest.124.1.328. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Harris B., Brodsky M. B., Michel Y., Lee F. -S., Walters B. Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. Journal of Speech, Language, and Hearing Research. (2007);50:585–594. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- 21.Martino R., Foley N., Bhogal S., Diamant N., Speechley M., Teasell R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke. (2005);36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 22.Monfils M. -H., Teskey G. C. Skilled-learning-induced potentiation in rat sensorimotor cortex: A transient form of behavioural long-term potentiation. Neuroscience. (2004);125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 23.Nicosia M. A., Hind J. A., Roecker E. B., Carnes M., Doyle J., Dengel G. A. Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. (2000);55:634–640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 24.Nourbakhsh M. R., Ottenbacher K. J. The statistical analysis of single-subject data: A comparative examination. Physical Therapy. (1994);74:768–776. doi: 10.1093/ptj/74.8.768. [DOI] [PubMed] [Google Scholar]
- 25.Pikus L., Levine M. S., Yang Y. X., Rubesin S. E., Katzka D. A., Laufer I. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. American Journal of Roentgenology. (2003);180:1613–1616. doi: 10.2214/ajr.180.6.1801613. [DOI] [PubMed] [Google Scholar]
- 26.Pouderoux P., Kahrilas P. J. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. (1995);108:1418–1426. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- 27.Robbins J., Gangnon R. E., Theis S. M., Kays S. A., Hewitt A. L., Hind J. A. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. (2005);53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 28.Robbins J., Gensler G., Hind J., Logemann J. A., Lindblad A. S., Brandt D. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: A randomized trial. Annals of Internal Medicine. (2008);148:509–518. doi: 10.7326/0003-4819-148-7-200804010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins J., Kays S. A., Gangnon R. E., Hind J. A., Hewitt A. L., Gentry L. R. The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation. (2007);88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbek J. C., Robbins J. A., Roecker E. B., Coyle J. L., Wood J. L. A penetration-aspiration scale. Dysphagia. (1996);11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe K., Ward L., Cichero J., Sopade P., Halley P. Thickened fluids and water absorption in rats and humans. Dysphagia. (2007);22:193–203. doi: 10.1007/s00455-006-9072-1. [DOI] [PubMed] [Google Scholar]
- 32.Steele C. M., Huckabee M. L. The influence of orolingual pressure on the timing of pharyngeal pressure events. Dysphagia. (2007);22:30–36. doi: 10.1007/s00455-006-9037-4. [DOI] [PubMed] [Google Scholar]
- 33.Steele C. M., Bailey G. L., Molfenter S. M. Tongue pressure modulation during swallowing: Water vs. nectar-thick liquids. Journal of Speech, Language, and Hearing Research. (2010);53:273–283. doi: 10.1044/1092-4388(2009/09-0076). [DOI] [PubMed] [Google Scholar]
- 34.Steele C. M., Bailey G. L., Molfenter S. M., Yeates E. M., Grace-Martin K. Pressure profile similarities between tongue resistance training tasks and liquid swallows. Journal of Rehabilitation Research and Development. (2010);47:651–660. doi: 10.1682/jrrd.2009.05.0068. [DOI] [PubMed] [Google Scholar]
- 35.Winstein C. J. Neurogenic dysphagia: Frequency, progression and outcome in adults following head injury. Physical Therapy. (1983);63:1992–1996. doi: 10.1093/ptj/63.12.1992. [DOI] [PubMed] [Google Scholar]
- 36.Winstein C. J., Rose D. K., Tan S. M., Lewthwaite R., Chui H. C., Azen S. P. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Archives of Physical Medicine and Rehabilitation. (2004);85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Youmans S. R., Youmans G. L., Stierwalt J. A. Differences in tongue strength across age and gender: Is there a diminished strength reserve? Dysphagia. (2009);24:57–65. doi: 10.1007/s00455-008-9171-2. [DOI] [PubMed] [Google Scholar]