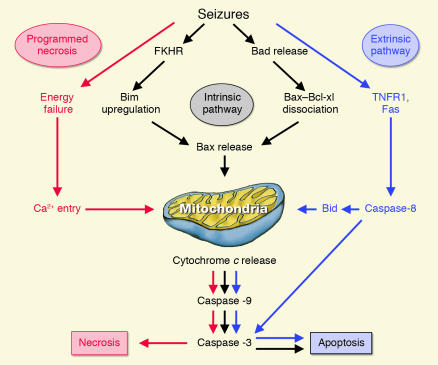
Figure 1.
Putative neuronal death pathways induced by epileptic seizures. In the “extrinsic” pathway of programmed cell death, activation of extracellular cell death receptors of the TNF superfamily (Fas and tumor necrosis factor receptor 1 [TNFR1]) induces activation of catalytic enzymes caspase-8 and caspase-3, causing widespread proteolytic damage and cell death. Mitochondria have a central role in the other forms of programmed cell death. In the “intrinsic” pathway, cytochrome c release is triggered by translocation of the proapoptotic factor Bax to the mitochondria. Seizures induce dephosphorylation of transcription factors FKHR and FKHRL-1, leading to the upregulation of the proapoptotic factor Bim, which forms an oligomer with Bcl-w, triggering Bax activation. Seizures may also induce dissociation of Bad from chaperone protein 14-3-3. Released Bad binds to Bcl-xl, provoking dissociation of Bax–Bcl-xl and Bax release. Bax penetrates the mitochondrial outer membrane and triggers cytochrome c release from the intermembrane space to the cytosol, where it activates caspase-9, which then activates caspase-3. Alternatively, cytochrome c release may be induced after Bid truncation by active caspase-8, linking the “extrinsic” and “intrinsic” pathways. In “programmed necrosis,” seizures deplete energy reserves, leading to mitochondrial calcium overloading, opening of the mitochondrial transition pore, and/or mitochondrial swelling and rupture of the outer membrane. Subsequent cytochrome c release activates the caspase cascade and leads to cell demise.