Abstract
Background:
Because of the conflicting results from previous studies regarding the efficacy of ginseng on lipid profile and anti-inflammatory and anti-proliferative activities of its components, we aimed to evaluate the effects of Panax ginseng on lipid profile, pro-oxidant – anti-oxidant status and high-sensitivity C reactive protein (hs-CRP) levels.
Methods:
Forty Iranian hyperlipidemic patients were randomly assigned to placebo (n = 20) or control (n = 20) groups in this double-blind randomized controlled trial. The ginseng or placebo was taken two capsules twice a day for 8 weeks. Total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), fasting blood glucose, serum creatinine and hs-CRP levels and pro-oxidant – anti-oxidant balance (PAB) were estimated before and after intervention.
Results:
There were no significant differences between the two groups with respect to changes in serum TG, LDL-C, HDL-C, and TC/HDL-C levels. Nor were there significant differences between the two groups with respect to changes in hs-CRP level and PAB from baseline to week 8.
Conclusions:
Our study demonstrates that ginseng does not have significant effects on lipid profile, Hs-CRP level and PAB. Further clinical studies, with a larger sample size, more prolonged period of therapy are needed to investigate the therapeutic effects of ginseng.
Keywords: High sensitivity C reactive protein, lipid profile, Panax ginseng, pro-oxidant – anti-oxidant status
INTRODUCTION
Panax ginseng is an important medicinal plant cultivated largely in Korea. The mature roots of these plants are used in many traditional medical formulations to treat several different conditions.[1] It has been used for more than 2000 years in Asia as a traditional medicine and dietary supplement.[2]
The primary active constituents of ginseng are thought to be the ginsenosides, which include more than 20 saponin triterpenes similar in structure to steroid hormones. These are believed to act via hormone receptors in the hypothalamus, pituitary glands, and other hormone sensitive tissues. Ginsenosides stimulate secretion of adrenocorticotropic hormone, leading to production of increased release of adrenal hormones, including cortisol. Ginsenosides have also been reported to stimulate ribonucleic acid (RNA) transcription, protein synthesis, and hepatic cholesterol production. In addition, they may stimulate synthesis of adrenal hormone precursors.[3,4]
According to recent studies, a hypolipidemic effect of Panax ginseng extract (PGE) is associated with decrease in total cholesterol (TC), triglycerides (TGs), low-density lipoprotein (LDL), Muscular Dystrophy Association (MDA) levels and an increase in high-density lipoprotein (HDL) level. Administration of PGE increased serum superoxide dismutase (SOD) and catalase (CAT) activities while decreased MDA level indicating that antioxidant potential of PGE might induce hypolipidemic effect as one of action mechanism.[4]
Evidence suggests that P. ginseng lowers cortisol levels in individuals with diabetes, while increasing cortisol levels in non-diabetic individuals.[3,4]
Specific triterpenoid saponins (diols) are claimed to cause improvements in endurance and learning. These compounds are also believed to contribute to sedative and antihypertensive properties. A second group (trials) has been reported to increases blood pressure and function as central nervous system stimulants. Ginsenosides have carbohydrate-sparing actions and may increase muscle endurance.[3,4]
The effects of P. ginseng have been reported to be dose-dependent. Low-doses increase blood pressure, while high doses exhibit a hypotensive effect.[3,4] Additionally, P. ginseng is reported to have immunostimulatory effects on the reticuloendothelial system. P. ginseng has also been reported to reduce weight loss and stabilize white blood cell counts during chemotherapy and to accelerate postsurgical recovery. P. ginseng helps the body adapt to stresses caused by chemotherapy and radiation.[3,4]
Many reports from clinical and experimental studies suggested that ginseng may have beneficial effects on reducing serum TC level and enhancing antioxidant status.[5,6]
However, Ismail et al. reported that a ginseng extract (G-115) had no significant hypolipidemic or antioxidant effects in rabbits.[7]
Administration of P. ginseng has minimal side effects.[8,9]
Ginsenosides, have been known for the anti-inflammatory and anti-proliferative activities by significant inhibitory effect on tumor necrosis factor-alpha.[10]
The most extensively studied biomarker of inflammation in cardiovascular diseases is C-reactive protein (CRP), for which standardized high-sensitivity assays (hs-CRP) are widely available.[11,12]
According to conflicting results from previous studies regarding the efficacy of ginseng on lipid profile and anti-inflammatory and anti-proliferative activities of its components, we aimed to evaluate the effects of P. ginseng on lipid profile, pro-oxidant – anti-oxidant status and hs-CRP level.
METHODS
This study was conducted as a double-blind randomized controlled trial. For the proposed study, to achieve a similar difference between the ginseng extract and placebo treatment groups with an 80% power and confidence interval of 95%, it is estimated that a sample size of 15 subjects per group will be required, that is, 30 in total. 40 patients were selected. The sample size was calculated according to the previous study of Kim, et al.[13] These patients referred to the cardiology clinic at Ghaem Hospital, Mashhad, Iran. No patients were on treatment with any medications. The inclusion and exclusion criteria were defined according to The American College of Cardiology (ACC) /The American Heart Association (AHA) guidelines. The inclusion criteria were: (1) Serum LDL-cholesterol (LDL-C) level 160-190 mg/dl in patients with 0-1 cardiovascular risk factors, (2) Serum LDL-C level 130-160 mg/dl in patients with >1 cardiovascular risk factors and <10% coronary heart disease (CHD) risk at 10 years according to Framingham risk scores, (3) Serum LDL-C level 100-130 mg/dl in patients with CHD equivalent risk factors and >20% CHD risk at 10 years according to Framingham risk scores, (4) TG level <200 mg/dl, (5) age >20 years.
The exclusion criteria were: (1) Overt hyperlipidemia requiring medical treatment (Serum LDL-C level >190 mg/dl, TG level <200 mg/dl); (2) past history of diabetes mellitus or fasting blood glucose level >126 mg/dl; (3) past history of documented cardiovascular disease; (4) pregnancy and lactation; (5) past history of renal failure or glomerular filtration rate <60 mL/min; (6) Acute infection, (7) Active bleeding with the exception of menses, (8) Taking anticoagulant; (9) psychosis or anxiety disorder; (10) mono amino oxidase inhibitors.
Of the total of 40 patients recruited and randomized to group, 20 participants were assigned to the placebo group, and 20 to the ginseng group. Patients visited the clinic over a period of 8 weeks at three stages (0, 1, and 2). At each clinic visit, height, weight, and body mass index (BMI) were measured for each participant. After allowing participants to rest for a minimum of 10 min, systolic and diastolic blood pressures were measured twice, and the averages were obtained. At stages 0 and 2, blood sampling and biochemical evaluation were undertaken. At stages 0 and 1, patients were administered the drug, or placebo [Figure 1]. For all the patients, we recommended the same diet according to ACC/AHA guidelines before intervention. Cardiac examinations also were done for all patients each stage. Patients were matched for confounding factors in both groups.
Figure 1.
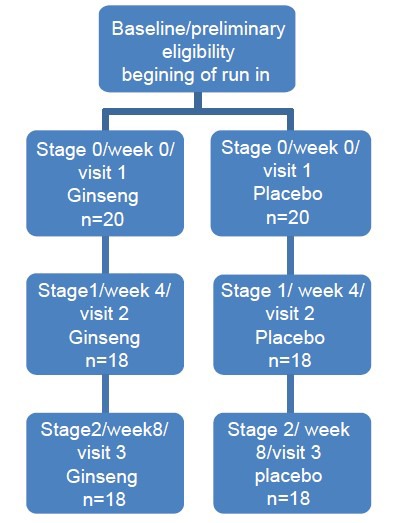
Study design and patient allocation design chart
Blood sampling was obtained before and after the 8 weeks’ drug/placebo administration. We choose an 8 week period according to the previous study of Kim et al.[4] After 12 h of fasting 10 mL of blood was collected from each patient.
Out of 10 mL, 5 mL of blood was collected into chilled tubes containing citrate and the rest into plain chilled tubes to obtain serum. Samples were centrifuged. Then, serum and plasma were frozen in dry ice prior to being stored at −80°C.
Blood samples were used for estimation of TC, TG, LDL-C, HDL-cholesterol (HDL-C), fasting blood glucose, serum creatinine and hs-CRP.
Pro-oxidants-anti-oxidants balance (PAB) assay was performed according to Alamdari DH et al. method.[14]
The standard solutions were prepared by mixing varying proportions (0-100%) of 1 mM hydrogen peroxide with 6 mM uric acid (in 10 mM NaOH).
The following standard solutions were prepared separately: Vitamin C (0-800 mM), Trolox (0-800 mM), uric acid (0-6 mM in 10 mM NaOH), glutathione (0-2500 mM), albumin (0-1000 mM [68 g/L]), hydrogen peroxide (0-1000 mM) and tert-buthylhydroperoxide (0-1000 mM).
Tetramethylbenzidine (TMB) solution was prepared by dissolving one TMB tablet in 10 mL of substrate buffer (0.05 mM phosphate citrate buffer, pH 5). For preparation of TMB cation, 18 mL of fresh chloramine T (10 mM) solution was added to 1 mL of TMB solution, incubated for 20 min; and 1.25 U of peroxidase enzyme solution was added to 9 mL of TMB solution. The working solution was prepared by mixing the two latter solutions, incubated for 5 min at room temperature and used immediately; 10 mL of each sample, standard or blank (distilled water) was mixed with 200 mL of working solution, in each well of a 96-well plate, which was then incubated in a dark place for 12 min at room temperature; at the end of the incubation time, 100 mL of 2N HCl was added to each well. The plate was then incubated for 45 min in a dark place and measured in an ELISA reader at 450 nm with a reference wavelength of 620 nm or 570 nm. A standard curve was provided from the values relative to the standard samples. The values of the PAB are expressed in arbitrary units, which are the percentage of hydrogen peroxide in the standard solution multiplied by 6. The values of the unknown samples were then calculated based on the values obtained from the above standard curve.
TC, TGs and HDL-C were measured using a standard enzymatic method by Biosystem kits and using an auto analyzer (BT 3000 model). LDL-C was calculated by Friedwald equation: LDL-C = TC - (TG/5 + HDL-C).
We measured hs-CRP using polyclonal antibodies against CRP coated onto latex participles. We measured it by Biosystem commercial kit with auto analyzer BT3000.
We measured FBS and serum creatinine using standard methods by Biosystem commercial kit with auto analyzer BT3000.
Ginseng extract was supplied by Gol Daru Co. Ltd., Tehran, Iran. Ginseng extract was provided as soft capsules. Each capsule contained 250 mg of ginseng extract, that contained 7 mg of ginsenosides and the components were characterized as follows: 2-3% for ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, Re, Rg1), panacene, beta sitosterol, low molecular weight polysaccharides, flavonoids. Ginsenosides or panaxosides are a class of steroid glycosides, and triterpene saponins, found exclusively in the plant genus Panax ginseng. Rb1 appears to be most abundant in Panax quinquefolius (American ginseng). Ginsenoside-Rc is a steroid molecule that can be found in the ginseng plant and is recognized for producing more sedative related results than other ginsenosides, such as ginsenoside-Re or ginsenoside-Rg. Rg1 appears to be most abundant in P. ginseng (Chinese/Korean ginseng).
The active (ginseng) group took two capsules twice a day for 8 weeks. The placebo was prepared in an industrial laboratory of the School of Pharmacy at Masshad University Medical School. We used a Granular powder included Lactose (66%), starch (33%) and Magnesium stearate (1%) as a placebo. The placebo was also taken twice a day for 8 weeks.
Statistical analysis was performed using SPSS version 16.0 for Windows. All data were presented as mean ± SD.
An independent t test was used to compare changes from baseline to 8 weeks between the two groups. In addition, a paired t test was used to compare baseline data with changes at 8 weeks for each group. The repeated measure ANOVA test was used to compare quantitative variables. Mann-Whitney and Chi-squared tests were used to compare ordinal and nominal qualitative variables, respectively. P <0.05 was considered significant for all data analyses.
The study, which was conducted according to the declaration of Helsinki, was approved by the Mashhad University of Medical Sciences Ethics Committee and all participants gave written informed consent.
Two participants in the control and one participant in the case group dropped out of the study because of failure to follow the protocol. One patient in the case group dropped out of study due to abnormal liver function test results. This did not have effect the power of the study substantially because we overestimate the sample size.
RESULTS
There was no significant difference between the two groups with respect to the occurrence of side effects (loss of appetite, diarrhea, headache, blurred vision, insomnia, agitation, rashes and bleeding).
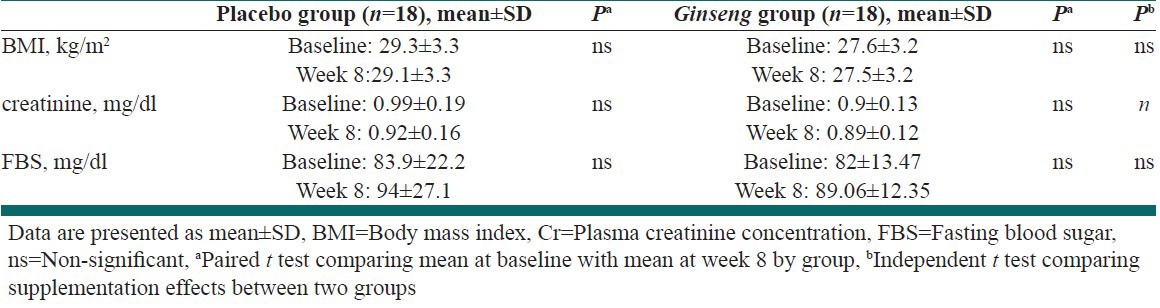
Table 1, shows the changes in BMI, creatinine, and fasting blood sugar from baseline to week 8 [Table 1].
Table 1.
Changes in body mass index, creatinine, fasting blood sugar from baseline to week 8
At the beginning of the study, there was no significant difference between the two groups regarding TC level. After 8 weeks of treatment, the serum TC level in the ginseng group decreased from 225.62 ± 44.41 mg/L to 184.8 ± 25.9 mg/L (P < 0.0001). However, there was no significant difference between the two groups with respect to the change in serum TC level [Table 2].
Table 2.
Changes in lipid profiles from baseline to week 8
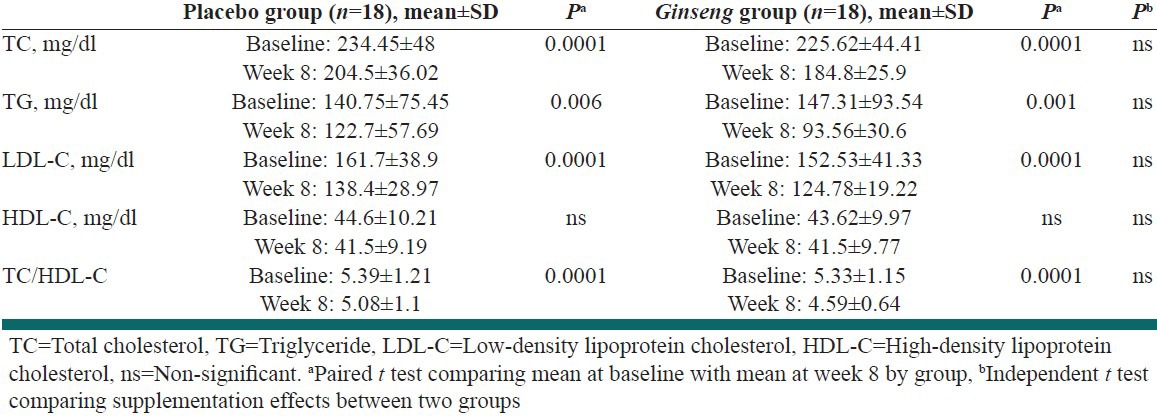
There were no significant differences between the two groups regarding TG, LDL-C, HDL-C, TC/HDL-C levels, at the beginning of study. After 8 weeks of treatment, the serum TG, LDL-C, HDL-C, TC/HDL-C levels in the ginseng group decreased from 147.31 ± 93.54 mg/L, 152.53 ± 41.33 mg/L, 43.6.7 ± 9.97 mg/L, 5.33 ± 38.91.15 mg/L to 93.56 ± 30.6 mg/L, 124.78 ± 19.22 mg/L, 41.5 ± 9.77, 4.5 ± 0.64 mg/L (P < 0.006, 0.0001, 0.173, 0.0001), respectively. However, there were no significant differences between the two groups with respect to the change in serum TG, LDL-C, HDL-C and TC/HDL-C levels [Table 2].
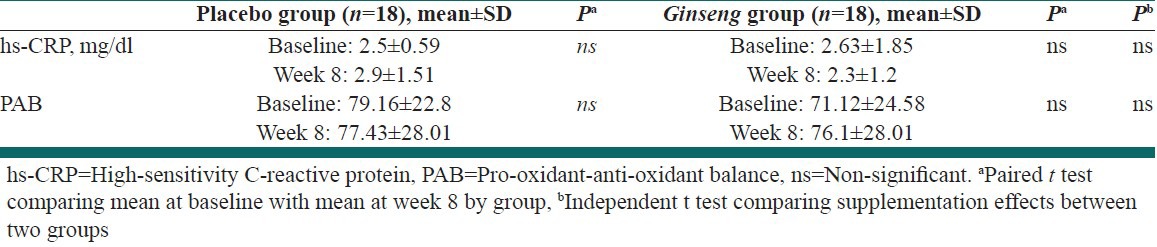
There were no significant differences between the two groups with respect to changes in serum hs-CRP and PAB from baseline to week 8 [Table 3].
Table 3.
Changes in high-sensitivity C-reactive protein, pro-oxidant-anti-oxidant balance from baseline to week 8
DISCUSSION
Our study was a randomized double blind controlled trial, which aimed to determine the effects of ginseng root extract on lipid profile, hs-CRP and PAB.
In the current study, 40 patients were recruited. Three participants dropped out of the study because of failure to follow the regimen. One patient in the ginseng group dropped out of study due to abnormal liver function test results.
No major side effects were detected, except the elevation of liver enzymes in one patient. After 1 month, one patient was found to have aminotransferase level more than twice the upper limit of normal. Aminotransferase level became normal after discontinuation of ginseng capsules. No previous study has reported this side-effect. Gastrointestinal side-effects were the most prevalent one. Surprisingly, gastrointestinal side-effects were more prevalent in the placebo group, but this was not statistically significant. Placebo contained lactose that could be responsible for gastrointestinal side-effects.
Overall, no statistically significant difference was detected between the case and control groups regarding side-effects.
According to Kim et al. study, ginseng showed significant effects on weight and fat mass reduction.[4] However, in our study, administration of ginseng had no significant effects on BMI and fat mass during the same period of treatment. This may be due to the greater sample size in our study. In addition, the dosage of PGE in our study was lower than Kim study.
Baseline demographic characteristics, lipid profiles, hs-CRP, creatinine level, fasting blood glucose were not significantly different between the two groups.
In our study, administration of ginseng significantly reduced TC, LDL-C, TG, TC/HDL-C levels during the period of study; however, there were no significant difference between the two groups with respect to the same effect.
Previous studies have demonstrated conflicting results regarding efficacy of ginseng on lipid profile. Kim et al. conducted a study that was not randomized and did not have control group. In this study, administration of ginseng at the dose of 8 g/day, reduced lipid levels.[4] In Sotaniemi et al. study, TG level decreased and HDL-C level increased after administration of 100-mg and 200-mg doses of ginseng but these effects were not statistically significant.[15] The result of this study is consistent with our own study. The sample size and study duration and ginseng dosage is similar to that used in our study.
In the study of Zuin et al., administration of a 40-mg dose of ginseng twice daily, had no effect on lipid profile.[16] This may due to low dosage of ginseng compared to our study. The duration of this study was longer than ours and the sample size was similar to our study.
Experimental and clinical evidences accumulated since 1990 have established inflammatory processes as important contributors to atherogenesis, as well as to the vulnerability of an atherosclerotic lesion to rupture or erosion.[11]
Based upon this evidence, protein markers of inflammation have been studied as non-invasive indicator of underlying atherosclerosis in apparently healthy individuals and of the risk of recurrent events in patients with established atherosclerotic vascular disease. The most extensively studied biomarker of inflammation in cardiovascular diseases is CRP.[9,10] CRP is an acute phase protein that is produced predominantly by hepatocytes under the influence of cytokines such as interleukin-6 and tumor necrosis factor-alpha.[17]
Data from more than 30 epidemiologic studies have shown a significant association between elevated serum or plasma concentrations of CRP and the prevalence of underlying atherosclerosis, the risk of recurrent cardiovascular events among patients with established disease, and the incidence of first cardiovascular events among individuals at risk for atherosclerosis.[18,19]
Ginsenosides, have been known for their anti-inflammatory and anti-proliferative activities by significant inhibitory effect on tumor necrosis factor-alpha, in vivo.[10] According to this evidence, we evaluated the correlation between administration of ginseng and the level of hs-CRP. Our results showed no significant difference between the case and control group with regard to hs-CRP level.
Previous studies showed a heightened state of oxidative stress following acute coronary syndrome and suggested that the PAB value may be considered as a cardiovascular risk predictor to estimate the extent of oxidative stress.[20,21] In our study, ginseng did not improve the PAB.
CONCLUSIONS
Our study demonstrates that ginseng does not appear to have any significant effects on lipid profile, hs-CRP level and PAB. We suggest further clinical studies, with greater sample size, more prolonged period of therapy to investigate the therapeutic effects of ginseng. The present study had several limitations. First, ginseng was administered at a dose of 500 mg/day for a limited period (8 weeks), and longer-term studies are necessary to show that this effect is sustained. Second, using several doses of ginseng could have been useful to determine whether the observed effects of ginseng are dose-dependent and whether higher doses exert more dramatic effects.
ACKNOWLEDGMENTS
We are particularly grateful to the patients and their family members who volunteered to participate in this study. This work was financially supported by Mashhad University of Medical Sciences, Mashhad, Iran. The results presented in this work have been taken from Morteza Manavifar's thesis in MUMS, with the following ID number: 89251.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 2.Kim ND. Pharmacologic effect of red ginseng. J Ginseng Res. 2001;25:2–10. [Google Scholar]
- 3.Chong SK, Oberholzer VG. Ginseng – Is there a use in clinical medicine? Postgrad Med J. 1988;64:841–6. doi: 10.1136/pgmj.64.757.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SH, Park KS. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–3. doi: 10.1016/s1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 5.Muwalla MM, Abuirmeileh NM. Suppression of avian hepatic cholesterogenesis by dietary ginseng. J Nutr Biochem. 1990;1:518–21. doi: 10.1016/0955-2863(90)90034-i. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Yan Y. The effect of ginsenosides of ginseng stem and leaf (GSL) on the lipid regulation and lipid peroxidation in chronic hyperlipidemic rabbits. Zhongguo Yaolixue Tonbao. 1991;7:110–6. In Chem Abstr 1992; 116:542s. [Google Scholar]
- 7.Ismail MF, Gad MZ, Hamdy MA. Study of the hypolipidemic properties of pectin, garlic and ginseng in hypercholesterolemic rabbits. Pharmacol Res. 1999;39:157–66. doi: 10.1006/phrs.1998.0421. [DOI] [PubMed] [Google Scholar]
- 8.Scaglione F, Pannacci M, Petrini O. The Standardised G115(R) Panax ginseng C.A. meyer extract: A review of its properties and usage. Evid-Based Integr Med. 2005;2:195–206. [Google Scholar]
- 9.Coon JT, Ernst E. Panax ginseng: A systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–44. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Choi K, Kim M, Ryu J, Choi C. Ginsenosides compound K and Rh (2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human astroglial cells. Neurosci Lett. 2007;421:37–41. doi: 10.1016/j.neulet.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: Implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47:418–25. [PubMed] [Google Scholar]
- 12.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 13.Kim SS, Park HY, Byun YH, Hwang BG, Lee JH, Shim YJ, et al. The effects of the blood lipid profile and body fat by long term administration of red ginseng product. J Ginseng Res. 2002;26:67–73. [Google Scholar]
- 14.Alamdari DH, Ghayour-Mobarhan M, Tavallaie S, Parizadeh MR, Moohebati M, Ghafoori F, et al. Pro-oxidant-antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem. 2008 Apr;41:375–80. doi: 10.1016/j.clinbiochem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–5. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 16.Zuin M, Battezzati PM, Camisasca M, Riebenfeld D, Podda M. Effects of a preparation containing a standardized ginseng extract combined with trace elements and multivitamins against hepatotoxin-induced chronic liver disease in the elderly. J Int Med Res. 1987;15:276–81. doi: 10.1177/030006058701500503. [DOI] [PubMed] [Google Scholar]
- 17.Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- 18.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 19.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 20.Ghayour-Mobarhan M, Alamdari DH, Moohebati M, Sahebkar A, Nematy M, Safarian M, et al. Determination of prooxidant – Antioxidant balance after acute coronary syndrome using a rapid assay: A pilot study. Angiology. 2009;60:657–62. doi: 10.1177/0003319709333868. [DOI] [PubMed] [Google Scholar]
- 21.Alamdari DH, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem. 2007;40:248–54. doi: 10.1016/j.clinbiochem.2006.10.017. [DOI] [PubMed] [Google Scholar]


