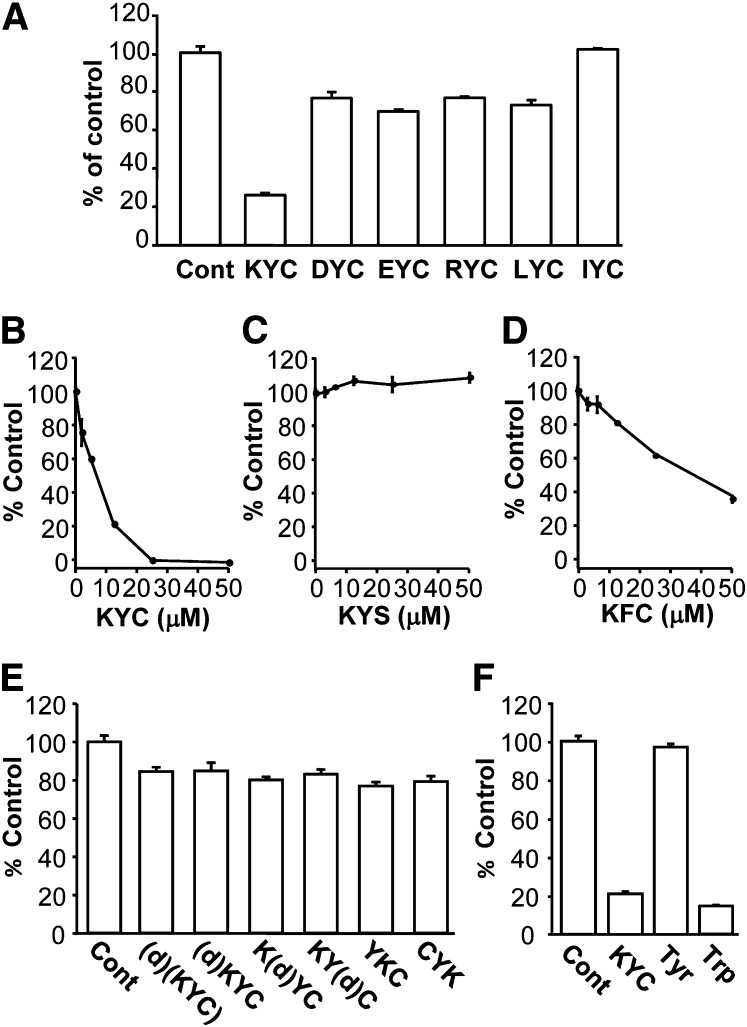
Fig. 1.
Effects of Tyr- and Cys-containing tripeptides on MPO-catalyzed HOCl formation. MPO (20 nM) was incubated with H2O2 (50 μM), NaCl (150 mM), taurine (5 mM), and various amounts of tripeptides in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) for 30 min. Reactions were halted by addition of catalase (2,000 units/ml). Taurine chloramine was quantified using the TMB assay. A: The effect of different tripeptides on the MPO-mediated HOCl formation. All tripeptide concentrations were at 12.5 μM. B–D: The dose-dependent inhibition of MPO-catalyzed HOCl formation by KYC (B), KYS (C), and KFC (D). KYC inhibited HOCl formation with an IC50 of ∼7 μM. E: Comparison of the effects of equimolar concentrations (12.5 μM) of KYC isomers on MPO-catalyzed HOCl formation. F: Comparison of the effect of equimolar concentrations (12.5 μM) of KYC with Tyr and Trp on MPO-catalyzed HOCl formation. All data (mean ± SD, n = 3) are expressed as percent of control (in the absence of tripeptides).