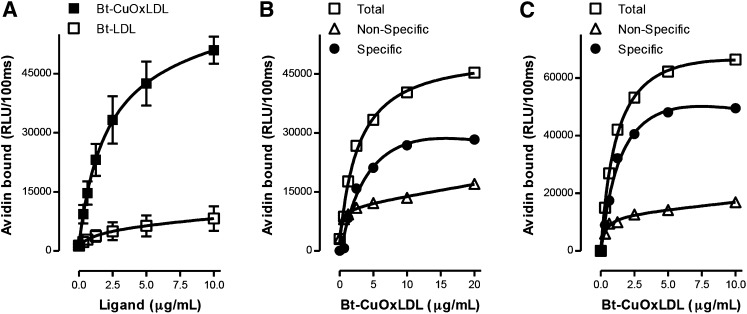
Fig. 1.
Total and specific binding of OxLDL to murine macrophages. In each panel, data are expressed as RLU/100 ms, and each point represents the average of triplicate determinations. A: Saturation binding curves of Bt-CuOxLDL and Bt-LDL to J774 macrophages. A total of 2.5 × 104 J774 macrophages/well were plated in 96-well culture plates and cultured for 72 h (see Materials and Methods). Cells were then washed and arrested on ice, and the media was replaced with ice-cold 1% BSA-PBS containing indicated amounts of Bt-CuOxLDL and Bt-LDL for 2 h. After extensive washing, the amount of biotinylated ligands bound to the plated cells was determined with the use of NeutrAvidin-alkaline phosphatase and a chemiluminescent ELISA technique as described in Materials and Methods. Data shown are mean ± SD of triplicate determinations from four independent experiments. B: Specific binding of CuOxLDL to J774 macrophages. Cells were plated and cultured in 96-well microtiter culture plates as in panel A. The cells were then washed and arrested on ice, and the media was replaced with 1% BSA-PBS containing indicated amounts of Bt-CuOxLDL in the absence or presence of a 20-fold excess of unlabeled CuOxLDL for 2 h. After extensive washing, the amount of Bt-CuOxLDL bound to the plated cells was determined as described in panel A. Specific binding was determined as the difference between total binding and binding in the presence of excess unlabeled CuOxLDL. Shown is one representative experiment of four. C: Specific binding of CuOxLDL to BMDM from Apoe−/− wt mice. After differentiation, 105 BMDM/well were plated in 96-well microtiter culture plates and incubated for 24 h (see Materials and Methods). Cells were washed and placed on ice, and then total and specific binding of OxLDL were determined as described in panel B. Data are from one representative experiment of two.