Abstract
Release of neurotransmitters and hormones by Ca2+ regulated exocytosis is a fundamental cellular/molecular process that is disrupted in a variety of psychiatric, neurological, and endocrine disorders. Therefore, this area represents a relevant target for drug and therapeutic development, efforts that will be aided by novel analytical tools and devices that provide mechanistically rich data with increased throughput. Toward this goal, we have electrochemically deposited iridium oxide (IrOx) films onto planar thin film platinum electrodes (20×300µm2) and utilized these for quantitative detection of catecholamine exocytosis from adrenal chromaffin cells trapped in a microfluidic network. The IrOx electrodes show a linear response to norepinephrine in the range of 0–400µM, with a sensitivity of 23.1±0.5mA/(M·mm2). The sensitivity of the IrOx electrodes does not change in the presence of ascorbic acid, a substance commonly found in biological samples. A replica molded polydimethylsiloxane (PDMS) microfluidic device with nanoliter sensing volumes was aligned and sealed to a glass substrate with the sensing electrodes. Small populations of chromaffin cells were trapped in the microfluidic sensing chamber and stimulated by rapid perfusion with high potassium (50mM) containing Tyrode’s solution at a flow rate of 1nL/s. Stimulation of the cells produced a rapid increase in current due to oxidation of the released catecholamines, with an estimated maximum concentration in the microfluidic device ~52µM. Thus, we demonstrate the utility of an integrated microfluidic network with IrOx electrodes for real-time quantitative detection of catecholamines released from small populations of cells.
Keywords: iridium oxide, electrochemistry, catecholamines, norepinephrine, microfluidic device, chromaffin cells
1. Introduction
Release of neurotransmitters by Ca2+ regulated exocytosis is the fundamental cellular/molecular process that underlies synaptic transmission and neuroendocrine hormone release. The finely tuned mechanisms that regulate neurosecretion are disrupted in a variety of psychiatric, neurological, and endocrine disorders, so are relevant targets for development of novel drugs and therapeutics aimed at improving human health. Hence, there is a need for a thorough, integrated understanding of stimulus-secretion coupling and reliable, high resolution methods to assay transmitter release and determine the neurosecretory phenotype in small populations of cells. Catecholamines including dopamine, norepinephrine, and epinephrine are one important class of neurotransmitter. Various approaches have been used to detect catecholamines including optical (chemical luminescence (Ragab et al., 2000), fluorescence (Nohta et al., 1997), spectrophotometric (Zhu et al., 1997)), electrophoresis (Jin et al., 1999), and liquid chromatography (Hows et al., 2004) methods. Electrochemistry represents one of the most attractive approaches due to the simplicity, high sensitivity, and temporal resolution of the method coupled with the ability to combine thin film electrodes with microfluidic technologies to engineer self contained lab-on-a-chip devices (Berberian et al., 2009; Lu et al., 2004). In recent years, many electrode modifications have been used for improving the determination of catecholamines such as conducting polymers (Liu et al., 2008), carbon nanotubes, (Bian et al., 2010; Tsai et al., 2008; Wang et al., 2004), diamond thin films (Dong et al., 2009), and other organic or inorganic compounds (Chen et al., 2006b; Chen et al., 2003; Chen et al., 2005; Sun et al., 2006).
In this manuscript, we report the characteristics of electrochemically deposited IrOx onto thin film platinum electrodes as a sensitive material for the detection of catecholamines. We also combine these planar sensors with a fluidic network to measure catecholamine secretion from small populations of chemically stimulated adrenal chromaffin cells at in-vivo like cell densities.
2. Materials and Reagents
Iridium tetrachloride, oxalic acid dehydrate, hydrogen peroxide (30% solution in water) and anhydrous potassium carbonate were purchased from Aldrich. Platinum (99,95%) rod diam. 2mm, and titanium (99,95%) rod diam. 2mm were purchased from Goodfellow Corp.. Universal pH buffers from VWR Scientific. The salts used for the Tyrode’s solution (potassium chloride, sodium chloride, magnesium chloride, sodium phosphate, sodium bicarbonate) were supplied by Fisher Scientific. Norepinephrine was analytical grade and came from Sigma. Polydimethylsiloxane (PDMS) elastomer composed of prepolymer and curing agent (Sylgard184 kit, Dow Corning, Midland, MI) was purchased from Essex Chemical. All chemicals were used as received.
3. Instrumentation
Thin film Ti-Pt electrodes were fabricated on microscope glass slides (24×76mm2) using e-beam vacuum evaporation (Inotec Corp.) of titanium and platinum from carbon crucible liners (Kurt J. Lesker Company). The deposition rate and the thickness of the films were monitored with a deposition controller MDC-360 (Maxtek, Inc.). A surface profiler (Alphastep200, Tencor Instruments) was used to measure the thickness of the photoresist and IrOx layers. An upright optical microscope (OLYMPUS BX-41) with a CCD camera Micropublisher 3.3, (QImagine, Canada) was used to monitor the surface morphology of platinum, iridium oxide thin films. A stereomicroscope STEMI 2000-C (Carl Zeiss, Germany) was used to align the iridium oxide thin film electrodes relative to the microfluidic network. An inverted microscope (AXIOVERT 25CFL, Carl Zeiss, Germany) in combination with a color CCD camera (KP-D20BU, Hitachi, Japan) was used to image the cells in the microfluidic device. The fluidic flow was controlled and maintained using a microsyringe pump controller MICRO-4 (WPI, Sarasota, FL) for flow rates ranging from 0.1–30nl/s. Electrochemical experiments were performed with a potentiostat (CHI 660B or CHI 1030) from CH Instruments (Austin, TX). A three-electrode configuration was used for the electrochemical deposition of the iridium oxide films and the quantification of catecholamine release from chromaffin cells. The counter electrode was either a Pt wire with a diameter of 1mm for beaker experiments or a thin Pt film for experiments in a microfluidic device. In all experiments a Dri-Ref-450 from WPI Inc. was used as reference electrode (diameter 0.45mm).
4. Experimental
In order to characterize the release of the catecholamine from chromaffin cells we utilized a microfluidic device which was fabricated according to the following steps: (i) deposition and patterning of the thin film Pt electrodes; (ii) electrochemical deposition of iridium oxide onto the Pt electrodes; (iii) replica molding of microfluidic devices with nanoliter cell culture volumes. Fig.1 depicts a schematic view of the electrode array with micron sized electrodes (Pt or IrOx films) and a T-type microfluidic channel network. In the next sections, we describe each of these fabrication processes in detail.
Fig.1.
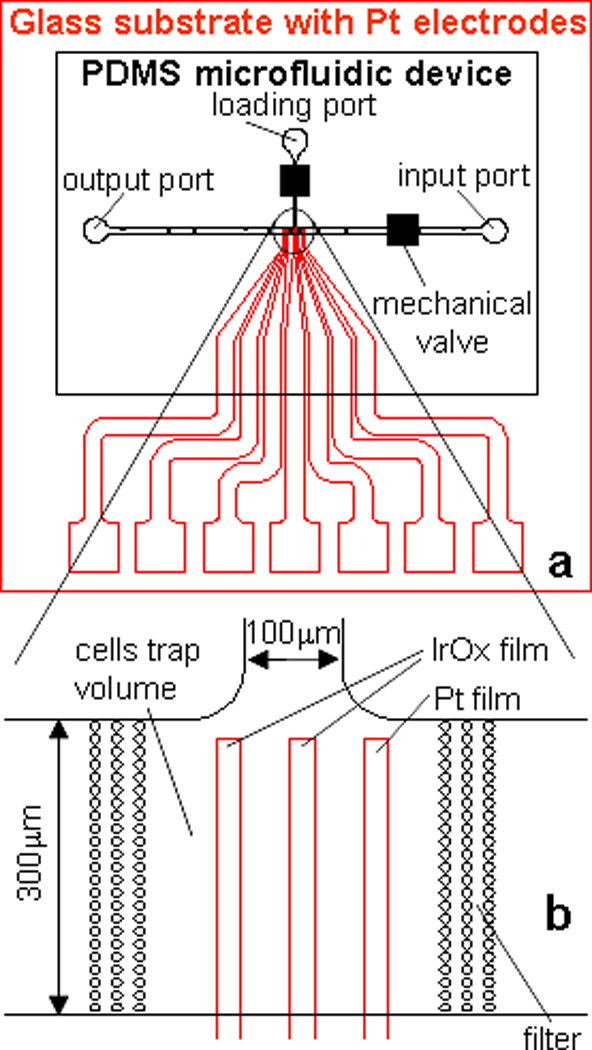
Schematic overview of the microfluidic device to measure catecholamine release from chromaffin cells in nanoliter volume. (a) black – PDMS microfluidic device with channel network; red – glass substrate with microelectrode array; (b) Enlarged view of catecholamine sensitive electrodes in the cell culture volume (CCV). The volume of CCV is ~3nL (300×300×30µm3), the size of catecholamine sensitive electrode is 20×300µm2, the gap in the filter post structure to retain the cells is 3µm.
4.1. Thin film conducting electrodes
The thin film conductive electrodes were fabricated on 25×25mm2 glass substrates. A Ti layer (~100Å) is used to mediate the adhesion of the Pt layer (~1000Å). The main parameters of the deposition process were: substrate temperature ~25°C; total background pressure during deposition ~3×10−7 torr (Ti) and 5×10−6 torr (Pt); the deposition rate for Ti and Pt was ~5Å/sec. The electrode array was patterned using standard photolithography. The exposed part of the Pt film was removed by ion beam etching. The photoresist was removed with acetone. Finally, the surface of the electrodes was cleaned with isopropyl alcohol, sonicated in DI water and electrochemically pre-treated by cycling the potential between −0.3 and +1.2 V versus Ag/AgCl in 0.5M sulphuric acid until a steady state voltammogram was obtained. A schematic drawing of the electrode geometry is shown in Fig.1a (red lines). The electrode sensing area was 100×300µm2 and 20×300µm2 for experiments in single channels and T-type microfluidic devices, respectively. Three to five central electrodes were used as catecholamine sensitive electrodes and lateral bare Pt electrodes were used as counter electrodes.
4.2. Iridium oxide electrode fabrication
The solution for the electrochemical deposition of iridium oxide films was prepared according to protocols described in the literature (Marzouk et al., 1998; Yamanaka, 1989). In our case we used the following protocol: 150mg iridium tetrachloride was dissolved in 100ml of DI water and stirred for 20min; 1ml of 30% hydrogen peroxide was added and the resulting solution was stirred for 15min; 0.5g of oxalic acid was added and stirred for another 5min; anhydrous potassium carbonate was added to adjust the pH to10.5. In fresh solution had a light yellow color which changed to light violet after two days. During this stabilization process the solution is kept in an open beaker at RT for 24 hours followed by storing the solution in a closed dark container at 4°C. Iridium oxide films were fabricated using galvanostatic electrochemical deposition. Light blue–dark blue, 100–150nm thick IrOx films (fig.1b) resulted from the deposition at a constant current density of 1–1.5mA/cm2 after 8÷10 min. The fresh iridium oxide films were conditioned in a universal buffer with a pH7.0 for two days to minimize potential drifts during measurements. Our anodically grown iridium oxide films typically show a Nernstain response with slopes between (−65)÷(−75)mV/pH at 20°C and linear responses in the pH range of 4–11.The deposition method and the characterization of the IrOx films is described further in our previous works (Ges et al., 2005; Ges et al., 2007).
4.3. Microfluidic network design
The microfluidic network was fabricated from PDMS (polydimethylsiloxane) using soft lithography. PDMS was prepared by mixing PDMS pre-polymer with curing agent and degassing the mixture in a vacuum chamber for 20min. The master was fabricated by spinning a 30µm thick layer of negative photoresist (SU-8 2025, MicroChem Corp, Newton, MA) on a silicon wafer and by exposing it to UV light (160mJ/cm2) through a chromium mask. A 30-minute post processing hard bake at 200°C was performed on a hot plate to increase the durability of the resist. The master was placed in a Petri dish, which was filled to a height of approximately 8mm with PDMS and cured in an oven for 3 hours at 70°C. After curing, the elastomer was mechanically separated from the master and cut into discrete devices. Access holes for the fluidic connections were punched into the PDMS using sharpened blunt 18 gauge needles.
The PDMS microfluidic device was manually aligned relative to the catecholamine sensitive electrodes (fig.1) with a stereo microscope. The PDMS device was sealed to the glass substrate by auto-adhesion, and stabilized with a mechanical clamp. Microsyringe pumps “Micro 4” (WPI, Sarasota, FL) were used to drive solution through the channels during the calibration of catecholamine sensors in the microfluidic devices.
It is known (Weibel et al., 2005) that in microfluidic devices, small pressure gradients could result in unpredictable flow pattern. We developed miniature mechanical screw shut-off valves for precise control of the flow in the microfluidic devices which were described in detail elsewhere (Ges et al., 2008). The valves were fabricated by drilling a pocket hole above the microfluidic channel and inserting an oversized threaded sleeve with screw into the hole. Turing the screw allowed us to compress the PDMS above the channel for closure. Our design allowed us to place two valves in close proximity to the cell trap volume (fig.1).
4.4. Integration of electrodes into the microfluidic device
Fig.1 shows the schematic overview of the microfluidic device to measure catecholamine release from chromaffin cells in nanoliter volumes. The electrode array consists of seven independent electrodes; the five central electrodes were coated with IrOx and used to measure catecholamines. The remaining bare electrodes were used as counter electrodes. Our design can be used to measure simultaneous catecholamine concentrations at multiple locations on chip or down-stream from the cell culture volume (CCV). We used two sizes of catecholamine sensitive electrodes with sensing areas of 100×500 and 20×800µm2. The distance between the electrodes was 2mm and 50µm, respectively.
We used a T-type channel configuration with a loading port and two perfusion ports. Two mechanical valves were inserted over the loading and the input channels to control the flow during measurements of catecholamine release. Chromaffin cells were loaded into the cell culture volume through the loading port using gravity or by applying a vacuum to the output port. The cells were confined by two filter structures (Fig.1b). The diameter of columns in the filter structure and the distance between columns was 10µm and 3µm, respectively. The cell culture volume was ~ 3 nL (300×300×30µm3).
4.5. Preparation of isolated chromaffin cells
Adult bovine adrenal glands were obtained from a local slaughterhouse and chromaffin cells were prepared by digestion with collagenase followed by density gradient centrifugation based on previously published protocols (Fenwick et al., 1978; Greenberg et al., 1982) The culture medium consisted of DMEM\F12 (1:1) supplemented with fetal bovine serum (10%), glutamine (2mM), penicillin/streptomycin (100 unit ml−1/100:gml−1), cytosine arabinoside (10:M) and 5-fluorodeoxyuridine (10:M). Fibroblasts were effectively suppressed with cytosine-arabinoside (10:M) (Sigma-Aldrich; St Louis MO), leaving relatively pure chromaffin cell cultures. A final volume of 5mL cell suspension was plated into T-25 culture flasks at a density of 0.7–0.8×106 cells/ml. Culture medium was replaced daily. For use on subsequent days the cells were resuspended from the tissue culture flasks to enable loading into the microfluidic chambers. To do so, cells on T25 tissue culture flasks were washed with calcium/magnesium free HBSS and then exposed briefly to 0.25% trypsin solution. Once cells loosened from the flask surface (1–2 minutes) an excess of culture medium was added, the cells were gently triturated and the cell suspension was filtered through a 40µm cell strainer. Following centrifugation the cell pellet was resuspended in physiological saline and again filtered through 40µm cell strainer to remove large clumps of cells prior to loading into the microfluidic device.
5. Results and discussion
The release of neurotransmitters by Ca2+ mediated exocytosis is a fundamental cellular/molecular process that underlies synaptic transmission and neuroendocrine hormone release. Catecholamines released from adrenal chromaffin cells play important physiological roles and mediate appropriate responses to acute stress (e.g. the fight-or-flight response). Chromaffin cells have also been widely used as a powerful cellular model to investigate the mechanisms and regulation of exocytotic transmitter release (Aunis et al., 1999; Bader et al., 2002; Burgoyne et al., 2003; Currie, 2010; Travis et al., 1998; Yoon et al., 2008). In this work, we describe the characteristics and use of electrochemically deposited IrOx on platinum thin film electrodes to investigate release of catecholamines from chromaffin cells in a microfluidic platform, which lends itself to future high-throughput screening approaches.
5.1. Characterization of sensitive electrodes
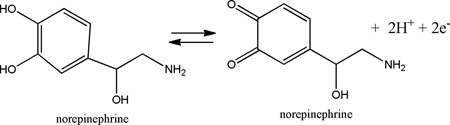
The electrochemical oxidation of norepinephrine (NE) occurs via the following half-reaction (Chen et al., 2003)
We investigated the electrochemical oxidative characteristics of norepinephrine at iridium oxide electrodes using cyclic voltammetry in a glass beaker filled with 25mL of phosphate buffer solution. The working electrode consisted of a thin film of iridium oxide, 0.1–0.15µm thick, on the glass substrate with a sensing area 20×500µm2, in combination with a Pt wire as counter and a commercially available reference electrode (DRI-Ref-450). Fig.2a shows the cyclic voltammogram of different norepinephrine concentrations in phosphate buffer solution (PBS, pH7) over the potential range −0.3 to 0.8V, at a sweep rate of 100mVs−1. The curves were obtained by successively adding a fixed amount of norepinephrine stock solution. The largest changes in the oxidation current for norepinephrine occurred at a potential difference of 0.72V, and scaled linearly with concentration (fig.2b). The slope of this relationship determines the sensitivity of the iridium oxide electrodes, and was typically 21.3±0.7mA/(M·mm2).
Fig.2.
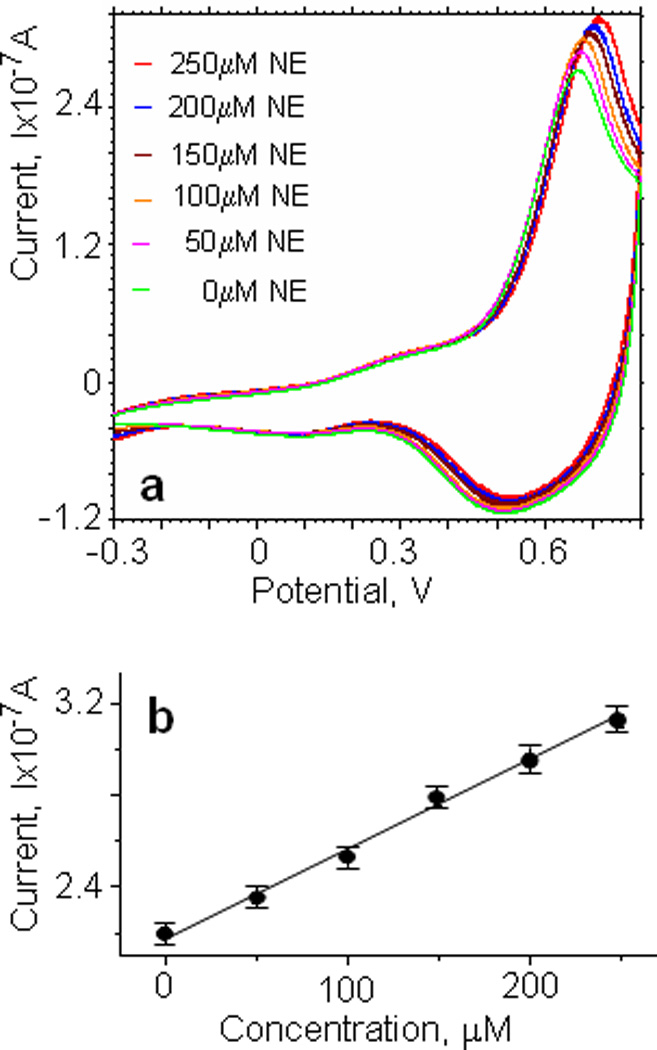
Detection of norepinephrine using iridium oxide electrodes in a “beaker experiment”. (a) Cyclic voltammograms using iridium oxide electrodes in PBS solution (pH 7) at various concentrations of norepinephrine in the range of 0–250µM. Scan rate 100mV/s. (b) Peak current as a function of norepinephrine concentration for iridium oxide electrode.
The oxidation peak potentials were observed at higher positive voltages with increasing scan rates in the range from 10–200mV/s (fig.3a). A linear relation between the amplitude of the oxidation peak current for norepinephrine and the square root of the scan rate (10–200mV/s) was obtained (fig.3a, insert), suggesting that the oxidation process of norepinephrine at the iridium oxide electrode was dominated by diffusion (Ren et al., 2006).
Fig.3.
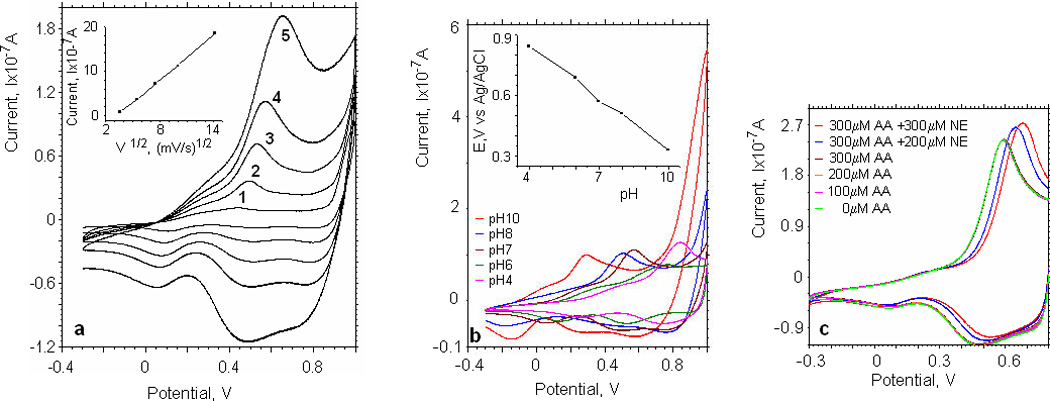
Electrochemical characterization of IrOx films: (a) Cyclic voltammograms of the iridium oxide electrode in PBS solution (pH 7) at various scan rates: (1) 10; (2) 30; (3) 60; (4) 100; (5) 200 mV/s. Inset: plot of anodic peak current vs (scan rate)1/2. (b) Cyclic voltammograms of the iridium oxide electrode at different pH values (the range of pH is 4–10). Scan rate 100mV/s; norepinephrine concentration 100µM. Inset: plot of anodic peak potential vs pH. (c) Cyclic voltammograms of the iridium oxide electrode in PBS solution (pH 7) at various concentration of ascorbic acid (the range 0–300µM) and norepinephrine (the range of 200–300µM). Scan rate 100mV/s.
Since iridium electrodes are also used as pH sensitive electrodes, we investigated the electrochemical response to 100mM norepinephrine in phosphate buffer solutions with different pH (4÷10) (fig.3b). The insert in fig.3b shows that the anodic peak potential decreased linearly with increasing pH, with the slope −79.5 mV/pH unit, which indicates a super Nernstian pH response. It can, therefore, be concluded that two electrons and two protons are involved in the electrode reaction of iridium oxide (Liu et al., 2008).
Ascorbic acid (AA) is a major interferant in the electrochemical detection of catecholamines. It is present in many biological fluids and is a widely used dietary supplement, prescribed for the treatment of common cold, mental illness, infertility, cancer etc (Chen et al., 2006a). Direct oxidation of ascorbic acid at conventional electrodes is irreversible and therefore requires a higher overpotential compared to the standard redox potential. Moreover, the direct reactions of ascorbic acid at conventional electrodes often produces a pronounced electrode fouling effect, which results in rather poor reproducibility (Chen et al., 2006a). To assess the impact of ascorbic acid on the electrochemical oxidative characteristics of the iridium electrodes, we performed measurements in the presence of ascorbic acid and norepinephrine. Fig.3c shows cyclic voltammograms obtained at different ascorbic acid and norepinephrine concentrations in PBS (pH 7) solution. Increasing the concentration of ascorbic acid did not change the position or value of the anodic peak current. Adding norepinephrine, we observed that the value of the oxidation peak increased. At a potential of 0.71V the current scales linearly with norepinephrine concentration and the sensitivity was not affected. Based on our results, we conclude that the presence of ascorbic acid does not significantly interfere with the electrochemical detection of norepinephrine using iridium oxide electrodes. Therefore iridium oxide electrodes might find a broad application for the measurements in catecholamines in blood and other biological fluids.
In order to investigate the characteristics of norepinephrine sensitive electrodes in a microfluidic environment, we filled a microfluidic channel (width 300µm) with different concentrations of norepinephrine in Tyrode’s solution and measured the CV characteristics (fig.4a, insert (II)). Fig.4a shows the cyclic voltammograms at different norepinephrine concentrations. The maximum oxidation current occurs at a potential of 0.74V (fig.4a, insert (I)) and is plotted as a function of the norepinephrine concentration in Fig.4a. Based on the slope, we computed a sensitivity of the iridium oxide electrode of 23.1±0.5mA/(M·mm2) for measurements in microfluidic environment. This current scale with area and therefore the sensitivities are comparable to our experiments in a beaker.
Fig.4.
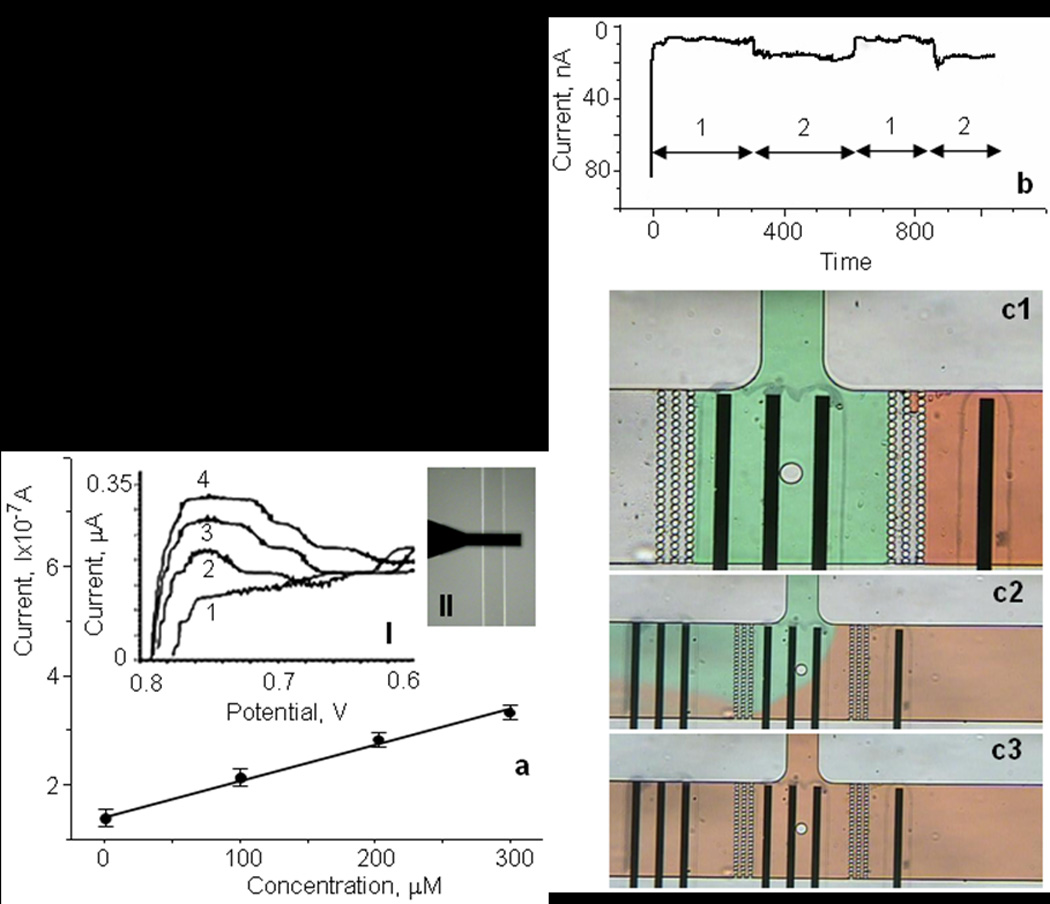
Characterization of IrOx electrodes in a microfluidic environment: (a) Current as a function of norepinephrine concentration for IrOx electrodes using Tyrode’s solution in the microfluidic environment. Inset:(I) Cyclic voltammograms of various concentrations of norepinephrine: (1) 0; (2) 100; (3) 200; (4) 300µM at a scan rate of 100mV/s. (II) Optical image of the microfluidic channel with IrOx electrode during calibration. (b) Calibration of the iridium oxide electrode (20×300µm2) in the microfluidic device with Tyrode’s solution at two different norepinephrine concentrations (0 and 100µM). (c1–c3) Optical images of the flow patterns in the microfluidic device during calibration with Tyrode’s solution without (green solution) and with (red solution) 100µm norepinephrine.
Figure 4b shows an example calibration of an iridium oxide electrode in response to solution exchange in a microfluidic device. Two Tyrode’s solution were used that contained either 100 µM norepinephrine (containing red food dye for visualization), or no added norepinephrine (green dye). The input and loading ports (see fig.1a) were used for delivery of buffer solution with different norepinephrine concentrations. As shown (Fig.1b and Fig.4b), our device architecture incorporates three rows of posts to confine cells in the cell culture volume (CCV). The diameter of posts and distance between rows was 10 and 3µm, respectively. This design allowed us to fill the CCV and the input channel with different buffer solutions separated by air trapped between the two rows of posts (Fig.4c1). The solutions in these two compartments do not mix and applying external pressure allows the rapid perfusion of the CCV with different solutions. The process consisted of the following steps: (a) filling the cell culture volume with buffer solution of known norepinephrine concentration; (b) close the loading channel with mechanical valve; (c) amperometric detection of norepinephrine at 0.74V; (d) open input valve and exchange the cell trap volume with buffer containing a different norepinephrine concentration. The fluidic exchange is visually demonstrated in fig.4c1–3. After applying external pressure to the input port the red solution passed through the air gap in the filter and filled the CCV with a different solution. The passing of air through CCV did not cause any interference with the amperometric detection of norepinephrine because the volume of air gap is small in comparison of the CCV. An exemplary amperometric current of an IrOx microelectrode in the microfluidic device in response to solution flow/exchange is shown in Fig.4b. We observed a stable oxidation current from the iridium oxide electrodes. Small current fluctuations might reflect residual flows or external noise/interference.
5.2. Catecholamine release from chromaffin cells trapped in the microfluidic device
Next we wanted to demonstrate the utility of the IrOx electrodes to detect catecholamine release from small populations of neurosecretory cells trapped in a microfluidic device. We chose to use adrenal chromaffin cells as these play important physiological roles through release of epinephrine and norepinephrine, and are widely used as a cellular model to investigate the regulation of exocytotic transmitter release (Aunis et al., 1999; Bader et al., 2002; Currie, 2010; Rettig et al., 2002). The layout of the configuration and the assembled device are shown in Fig.1 and Fig.5b. In order to operate the catecholamine sensitive electrodes in microfluidic environment, we placed a counter electrode in the input channel and a reference electrode in the output port. Three iridium oxide electrodes (20×300µm2) were situated inside the cells trap volume. Having 3 sensitive electrodes in CCV affors the ability to measure spatially resolved catecholamine release using a multichannel potentiostat. To trap the chromaffin cells in the CCV, we used the 3mL loading port as a cell reservoir. A concentrated cell suspension (~2mL) was added to the loading port using a syringe. A combination of a negative pressure gradient in the output port and gravity allowed us to load the cells into the CCV within 3–10min after injecting cells into the loading port. In a typical experiment the CCV was occupied with a single layer of chromaffin cells at coverages of 80–95% (Fig.5b). After cell seeding, the mechanical valve was used to close the loading channel blocking any residual flow.
Fig.5.
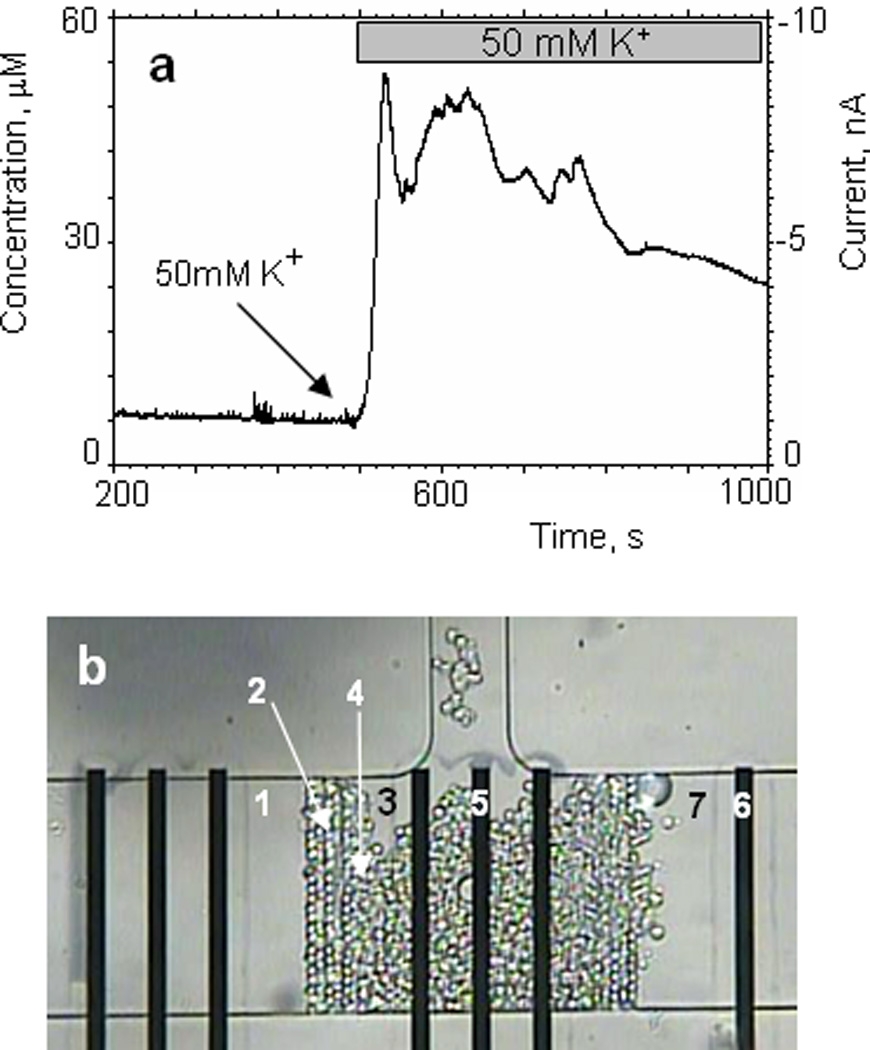
Detection of catecholamine release from a small population of chromaffin cells trapped in the microfluidic device. Secretion of catecholamines was evoked by exposure to high K+.
(a) oxidation current is plotted against time during the stimulation of chromaffin cells in the microfluidic device with Tyrode’s solution containing 50mM of potassium. The perfusion rate was 1 nL/s. (b) optical image of the chromaffin cells trapped in the microfluidic device during the experiment. (1) microfluidic channel; (2) filter; (3) cells culture volume; (4) chromaffin cells; (5) working electrode – IrOx; (6) counter electrode – Pt; (7) 50mM K+ solution separated by an air gap.
Secretion of catecholamines can be triggered by exposing the chromaffin cells to elevated extracellular potassium (50mM) which depolarizes the membrane potential, opens voltage-gated Ca2+ channels, and results in Ca2+ influx that triggers exocytosis (Sun et al., 2006). First we confirmed that the elevated [K+] solution does not interfere with catecholamine detection by the IrOx electrodes. In the supplemental data we show the response of the iridium oxide electrode in the microfluidic device to norepinephrine (200µM) was not altered in the presence of elevated (50mM) potassium. Quantification of potassium-evoked catecholamine secretion consisted of the following steps: (a) filling the input channel with a 50mM KCl solution separated by a small air gap in the filter from the CCV and closing the input channel with the mechanical valve; (b) loading chromaffin cells into the CCV through the loading port and closing the loading channel ; (c) the baseline recordings in Tyrode’s solution with nominal [K+] (5mM); (d) opening the mechanical valve in the input channel and perfusing a high K+ solution through the CCV using a syringe pump connected to the input port; (e) recording the amperometric current due to released catecholamines. The temporal profile of catecholamine release from chromaffin cells during exposure to a high K+ solution is shown in Fig.5a. The current reaches a stable value during baseline recording and does not change significantly during the initial part of experiment. After 500s we started to perfuse the CTV with Tyrode’s solution containing 60mM [K+] at a flow rate of 1nL/s. We observed a rapid increase in current due to oxidation of catecholamines released from the chromaffin cells. The current amplitude reflects the amount of catecholamine released from the cell population into the microfluidic chamber. Taking into account the sensitivity of the iridium oxide electrode (23mA/M·mm2) we estimated that the concentration of catecholamines was ~52µM.
6. Conclusions
We have electrochemically deposited IrOx films onto planar thin film platinum electrodes with working areas of 20×300µm2 and used these to quantify evoked catecholamine secretion from small populations of chromaffin cells trapped in a microfluidic device. The IrOx electrodes show a linear response to norepinephrine in the range of 0–400 µM with a sensitivity of 23.1±0.5mA/(M·mm2), and this was not altered by ascorbic acid. We anticipate future integration of these electrodes into various microfluidic device designs. This will provide key enabling technology, for example for high-content, increased throughput assessment of the neurosecretory phenotype of small populations of cells from transgenic mice, and of novel drugs or therapeutics with low compound utilization.
Supplementary Material
Influence of the 50mM KCl Tyrode’s solution on the current response of the iridium oxide electrodes in comparison to the response to Tyrode’s solution without high K+. Inset: optical image of the microfluidic device during the experiment; green – Tyrode’s solution, red – Tyrode’s solution with 50mM KCl.
Acknowledgments
We are grateful to Sarah McDavid and Mark Jewell for chromaffin cell preparation and for refining cell isolation and culture for loading into the microfluidic chambers. We also thank Brad Lubbers for helpful discussions. This work has been supported in part by NIH Grant U01AI061223, the Vanderbilt Institute for Integrative Biosystems Research and Education, and the NIH, NINDS Grant R01NS052446.
References
- Aunis D, Langley k. Acta Physiol Scand. 1999;167:89–97. doi: 10.1046/j.1365-201x.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- Bader MF, Holz RW, Kumakura K, Vitale N. Ann.NY Acad.Sci. 2002;971:178–183. doi: 10.1111/j.1749-6632.2002.tb04461.x. [DOI] [PubMed] [Google Scholar]
- Berberian K, Kisler K, Fang Q, Lindau M. Anal.Chem. 2009;81:8734–8740. doi: 10.1021/ac900674g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian C, Zeng Q, Xiong H, Zhang X, Wang S. Bioelectrochem. 2010;79:1–5. doi: 10.1016/j.bioelechem.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Physiol.Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Chen SM, Chen JY, Vasantha VS. Electrochim. Acta. 2006a;52:455–465. [Google Scholar]
- Chen SM, Liao CJ, Vasantha VS. J. Electroanal. Chem. 2006b;589:15–23. [Google Scholar]
- Chen SM, Liu MI. J. Electroanal. Chem. 2005;579:153–162. [Google Scholar]
- Chen SM, Peng KT. J. Electroanal. Chem. 2003;547:179–189. [Google Scholar]
- Currie KP. Cell Mol.Neurobiol. 2010;30:1201–1208. doi: 10.1007/s10571-010-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wang S, Liu A, Galligan JJ, Swain GM. J. Electroanal. Chem. 2009;632:20–29. [Google Scholar]
- Fenwick EM, Fajdiga PB, Howe NB, Livett BG. J.Cell Biol. 1978;76:12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ges IA, Dzhura IA, Baudenbacher FJ. Biomed. Microdev. 2008;10:347–354. doi: 10.1007/s10544-007-9142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ges IA, Ivanov BL, Schaffer DK, Lima EA, Werdich AA, Baudenbacher FJ. Biosens. Bioelectron. 2005;21:248–256. doi: 10.1016/j.bios.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Ges IA, Ivanov BL, Werdich AA, Baudenbacher FJ. Biosens. Bioelectron. 2007;22:1303–1310. doi: 10.1016/j.bios.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Greenberg A, Zinder O. Cell Tissue Res. 1982;226:655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Hows MEP, Lacroix L, Heidbreder C, Organ AJ, Shah AJ. J. Neurosci. Meth. 2004;138:123–132. doi: 10.1016/j.jneumeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Jin W, Jin L, Shi G, Ye J. Anal. Chim. Acta. 1999;382:33–37. [Google Scholar]
- Liu AL, Zhang SB, Chen W, Lin XH, Xia XH. Biosens. Bioelectron. 2008;23:1488–1495. doi: 10.1016/j.bios.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Lu LP, Wang SQ, Lin XQ. Anal. Chim. Acta. 2004;519:161–166. [Google Scholar]
- Marzouk SAM, Ufer S, Buck RP, Johnson TA, Dunlap LA, Cascio WE. Anal.Chem. 1998;70:5054–5061. doi: 10.1021/ac980608e. [DOI] [PubMed] [Google Scholar]
- Nohta H, Yukizawa T, Ohkura Y, Yoshimura M, Ishida J, Yamaguchi M. Anal. Chim. Acta. 1997;344:233–240. [Google Scholar]
- Ragab GH, Nohta H, Zaitsu K. Anal. Chim. Acta. 2000;403:155–160. [Google Scholar]
- Ren W, Luo HQ, Li NB. Biosens. Bioelectron. 2006;21:1086–1092. doi: 10.1016/j.bios.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Rettig J, Neher E. Science. 2002;298:781. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- Sun X, Gillis KD. Anal.Chem. 2006;78:2521–2525. doi: 10.1021/ac052037d. [DOI] [PubMed] [Google Scholar]
- Travis ER, Wightman RM. Annu.Rev.Biophys.Biomol.Struct. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Yang CC, Shih PY, Wu CS, Chen CD, Pan CY, Chen YT. J.Phys.Chem.B. 2008;112:9165–9173. doi: 10.1021/jp803000a. [DOI] [PubMed] [Google Scholar]
- Wang G, Liu X, Yu B, Luo G. J. Electroanal. Chem. 2004;567:227–231. [Google Scholar]
- Weibel DB, Kruithof M, Potenta S, Sia SK, Lee A, Whitesides GM. Anal.Chem. 2005;77:4726–4733. doi: 10.1021/ac048303p. [DOI] [PubMed] [Google Scholar]
- Yamanaka K. Jap.J. Appl. Phys. 1989;28:632–637. [Google Scholar]
- Yoon EJ, Hamm HE, Currie KP. J.Neurophysiol. 2008;100:2929–2939. doi: 10.1152/jn.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Huang X, Li J, Shen H. Anal. Chim. Acta. 1997;357:261–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Influence of the 50mM KCl Tyrode’s solution on the current response of the iridium oxide electrodes in comparison to the response to Tyrode’s solution without high K+. Inset: optical image of the microfluidic device during the experiment; green – Tyrode’s solution, red – Tyrode’s solution with 50mM KCl.


