Fig. 6.
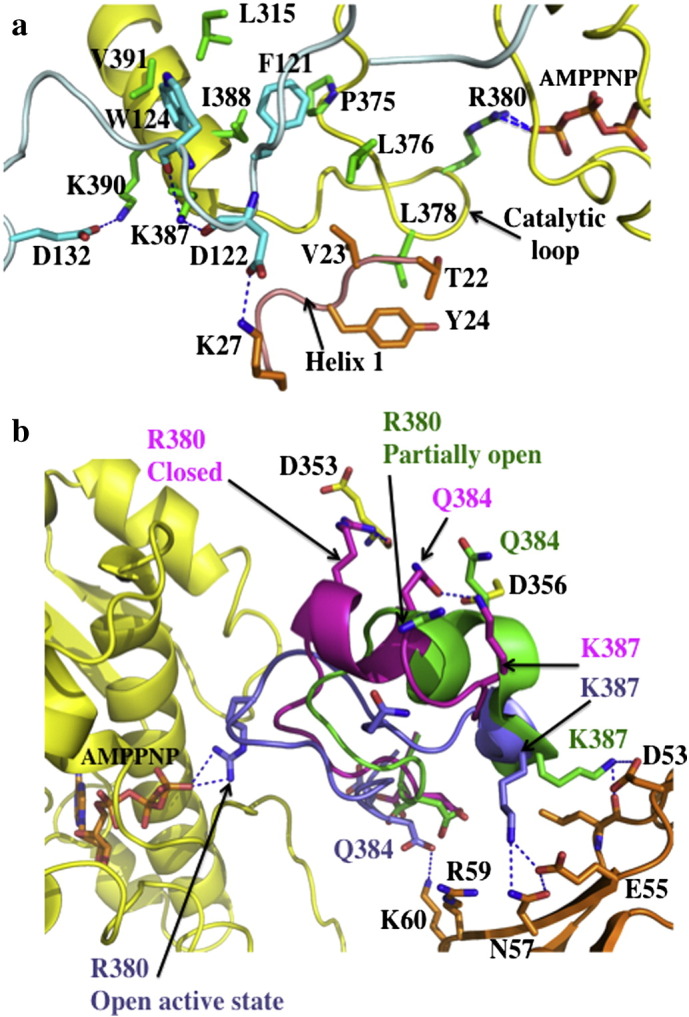
ATPase modulated states of Hsp90. a), The ‘inhibited’ state of Sba1 bound Hsp90 (pdb, 2CG9). Sba1 is shown in light blue with amino acid residues in cyan. The middle domain of Hsp90 is shown in yellow with amino acids residues in green. The N-terminal domain sequences from the neighboring Hsp90 monomer are shown in gold. ATP is shown bound to Arg 380 by hydrogen bounds (dotted blue lines). Trp 124 of Sba1 binds into a hydrophobic pocket formed by the Hsp90 residues, Leu 315, Ile 388 and Val 391, which signals the catalytic loop to be released into its active open conformation. This state represents an ‘inhibited’ form of Hsp90 that is committed to hydrolyzing ATP. b), Modulation of the catalytic loop of Hsp90. Hsp90 is shown in yellow (pdb2CG9; middle- and C-terminal-domain not shown). The catalytic loop is shown in magenta (closed state; pdb, 1HK7), green (partially open state of the N-domain Aha1 — middle domain of Hsp90 complex, pdb 1USU) and the open active state (blue) of the Sba1-bound structure (pdb 2CG9). Aha1 is in gold. The partially open state of the catalytic loop in the N-Aha1 — middle-Hsp90 complex results because in this structure Arg 380 cannot interact with ATP. Hydrogen bonds are shown as dotted blue lines.
