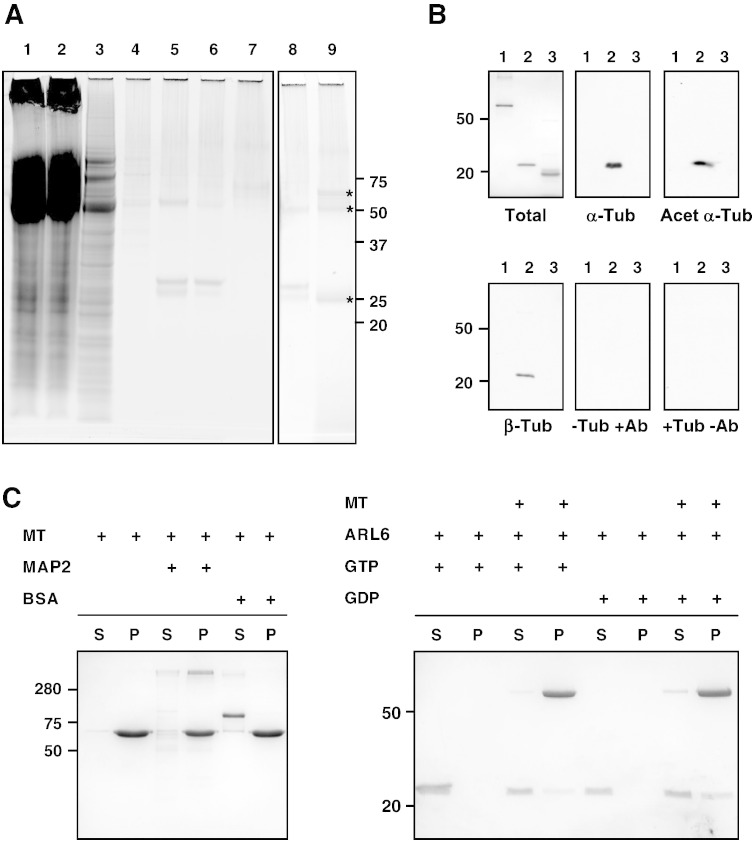
Fig. 4.
Identification of tubulin as an interacting partner of T. brucei ARL6. (A) PTP purification of TbARL6. Samples were separated by SDS-PAGE and stained with Coomassie. 1, Input material (0.05% of fraction loaded), 2, 3, 4, Flowthrough and washes of IgG Sepharose column (0.05%, 0.2%, and 0.13%), 5 and 8, TEV protease eluate (1%), 6, Flowthrough of the anti-ProtC matrix (0.3%), 7, Flowthrough of StrataClean resin (2.9%), 9, Final eluate (100%). The final eluted fraction contains three major protein bands (marked by asterisks) which were identified as protein C-tagged TbARL6 (23 kDa), α/β-tubulin (50 kDa) and a keratin contaminant (~ 65 kDa). (B) Tubulin overlay assay. Purified recombinant proteins (1 μg per lane) were separated by SDS-PAGE and either stained with Coomassie (Total) or transferred to PVDF (remaining panels). 1, Human Ras (R9894, Sigma), 2, TbARL6, 3, bovine serum albumin (New England Biolabs). Blots were incubated in tubulin solution overnight, then probed with mouse monoclonal antibody TAT1 against α-tubulin (α-Tub), monoclonal antibody clone 6-11B-1 which specifically recognises acetylated α-tubulin (Acet α-Tub) or monoclonal antibody TUB 2.1 against β-tubulin (β-Tub). Control immunoblots were processed with TAT1 antibody but without tubulin (− Tub + Ab) or without primary antibody (+ Tub − Ab). (C) Microtubule binding protein assay. Purified proteins were incubated with a microtubule preparation before ultracentrifugation. Soluble (S) and pellet (P) fractions were subjected to SDS-polyacrylamide electrophoresis and stained with Coomassie. MT, microtubules. MAP2, Microtubule associated protein 2 (280 kDa, positive control), BSA, bovine serum albumin (68 kDa, negative control). Corresponding protein marker positions are shown (kDa).