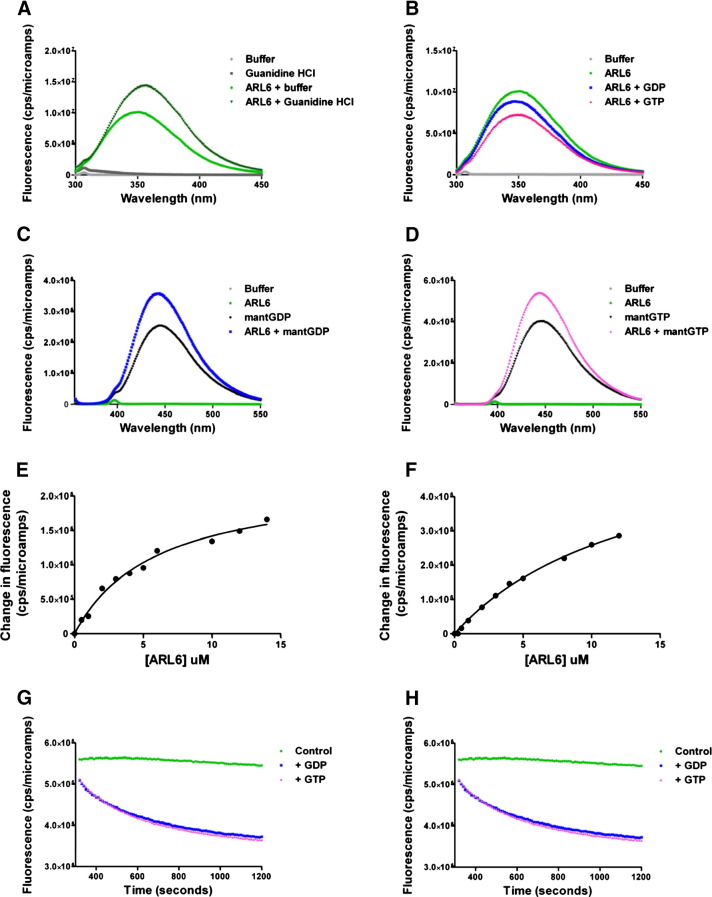
Fig. 5.
T. brucei ARL6 nucleotide interactions. Purified recombinant TbARL6 was used at 5 μM in 50 mM phosphate buffer unless otherwise stated. (A) Tryptophan fluorescence emission spectra of TbARL6 in the presence and absence of 6 M guanidine hydrochloride. (B) Tryptophan fluorescence emission spectra of TbARL6 in phosphate buffer and 10 mM MgCl2 in the presence and absence of 12.5 μM unlabelled GDP or GTP. (C, D) Mant fluorescence emission spectra of mantGDP (C) and mantGTP (D) in the presence and absence of TbARL6 protein. (E, F) Dissociation constants (Kd) for TbARL6 and mantGDP (E) or mantGTP (F) were calculated by non-linear regression (one site specific binding) from data obtained at a single emission wavelength (GDP, 445 nm, GTP, 447 nm) with a fixed concentration of nucleotide and a range of protein concentrations. (G, H) Guanine nucleotide exchange by TbARL6. Protein was prebound to mantGDP (G) or mantGTP (H), then mixed at 300 s with a large excess of unlabelled nucleotide. The decrease in fluorescence was recorded over time and dissociation rate constants (κdiss) calculated by non-linear regression (one phase exponential decay). Controls represent protein and mant-labelled nucleotide without the addition of unlabelled nucleotide. All data are representative of at least 3 independent experiments.