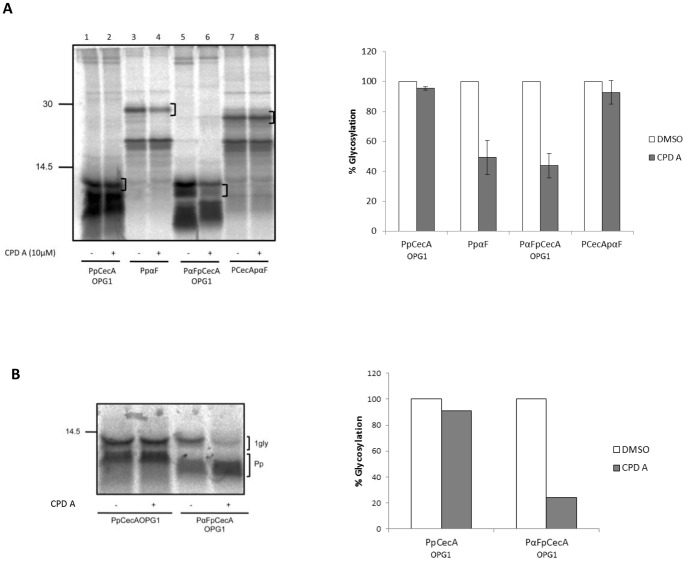
Figure 5. The signal sequence modulates post-translational interactions with Sec61.
A. PpCecAOPG1, PpαF and the two chimeras, PαFpCecAOPG1 and PCecApαF were translated in the presence of ER microsomes and either DMSO (−) or 10 µM CPD A (+). Membranes were isolated by centrifugation and associated proteins were analyzed by SDS-PAGE and phosphorimaging. A bracket indicates the predominant glycoform, and this species was quantified and expressed as a percentage of the paired DMSO treated samples which was set to 100%. The values shown are means (±s.e) for n = 3. In this experiment, PαFpCecAOPG1 has generated two products upon translocation into the ER, both of which are reduced upon treatment with CPD A concomitant with an apparent increase in the prepro-form of the protein that retains its signal sequence (cf. lanes 5 and 6). PαFpCecAOPG1 has only a single N-linked glycan and the two forms observed are most likely a consequence of glucose trimming [40]. B. The post-translational translocation of PpCecAOPG1 and PαFpCecAOPG1 into ER derived microsomes pretreated with DMSO (−) or CPD A dissolved in DMSO (+) was performed by adding membranes after protein synthesis had been terminated using puromycin (see also materials and methods) and then processing the samples as described for A. Glycosylated products shown in panel B were quantified by phosphorimaging and expressed as a percentage of their paired DMSO sample.