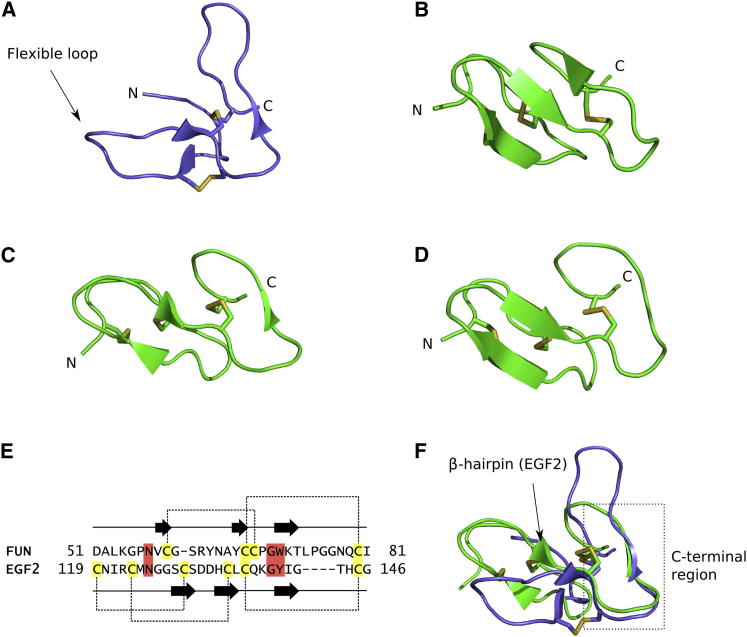
Figure 4.
Domain Structures in the FUN-EGF3 Fragment
(A) The FUN domain comprises two loops (one of which is flexible) linked by two disulphide bonds in a 1-3, 2-4 pattern (C59-C68 and C67-C80).
(B) The EGF1 domain has a canonical 1-3, 2-4, 5-6 disulphide bond arrangement and a three-stranded antiparallel β sheet.
(C and D) Structure of the EGF2 (C) and EGF3 (D) domains, which contain a β-hairpin, is shown.
(E) Comparison of the FUN and EGF2 primary structure. Sequences were aligned manually on the basis of structural homology. Arrows represent stretches of β-sheet-like secondary structure and dotted lines indicate disulphide bonds.
(F) Backbone of the EGF2 domain (green) superimposed on the FUN domain (lilac), illustrating the similarity of their C-terminal portions (dashed box). The β-hairpin in EGF2 is indicated.