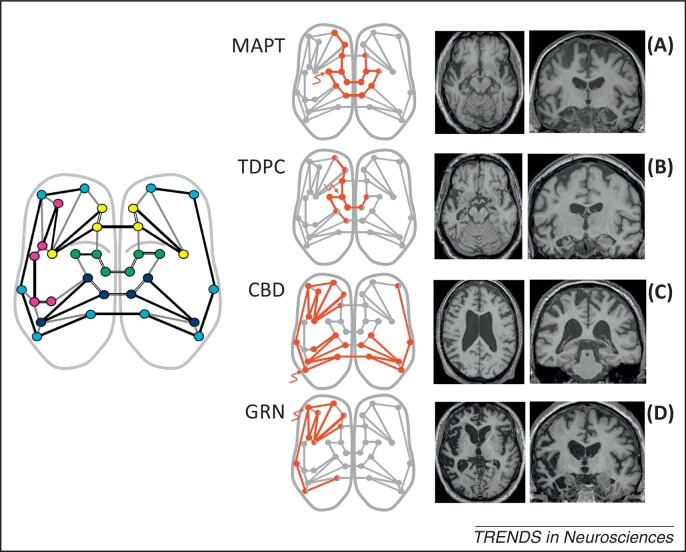
Figure 2.
Scaling nexopathies to large-scale brain networks. The inset cartoon (left) shows a stylised axial view of the cerebral hemispheres in a normal brain. Circles represent neural network elements and colours code large-scale functional networks associated with generic clinical syndromes in previous connectivity work [7]: the anterior temporal lobe semantic network (green); the frontoinsular ‘salience’ network implicated in behavioural variant frontotemporal dementia (yellow); the dominant hemisphere speech production network implicated in progressive nonfluent aphasia (magenta); the frontoparietal network associated with corticobasal syndrome (light blue); and the temporoparietal ‘default mode’ network implicated in Alzheimer's disease (dark blue). Putative shorter-range clustered (unfilled black lines) and longer-range distributed (filled black lines) connections between network elements are shown; connections between major functional networks are also represented (grey lines). The middle panels show proposed cross-sectional schemas of network breakdown (red, following Figure 1, main text), after an instigating insult (wavy arrow) associated with a pathogenic protein. Alongside each panel, axial and coronal MRI brain sections show corresponding observed atrophy profiles in patients with representative, canonical, pathologically confirmed, proteinopathies (CBD, corticobasal degeneration associated with 4-repeat tau pathology; GRN, mutation in progranulin gene; MAPT, mutation in microtubule-associated protein tau gene; TDPC, TDP-43 type C pathology [19], associated with the clinical syndrome of semantic dementia; the left hemisphere is on the left in all sections). These atrophy profiles illustrate macroanatomical scaling of the nexopathy templates proposed in Figure 1 (main text): (A) predominant involvement of clustered (shorter-range) connections with uniform extension, leading to relatively focal (temporal lobe) atrophy that is relatively symmetrically distributed between the cerebral hemispheres; (B) predominant involvement of clustered (shorter-range) connections with a strong gradient of network damage, leading to relatively focal (temporal lobe), strongly asymmetric atrophy; (C) predominant involvement of distributed (longer-range) connections with uniform extension, leading to distributed, relatively symmetrical atrophy; and (D) predominant involvement of distributed (longer-range) connections with a gradient of network damage, leading to distributed, strongly asymmetric atrophy. A particular proteinopathy here affects network connections with particular characteristics (e.g., clustered versus distributed synaptic linkages); functional networks will be targeted according to their specific network characteristics, but the effects of a particular nexopathy will in general tend to spread between functional networks, while continuing to target connections with similar properties across these networks. This would account for empirical variability in the closeness with which proteinopathies map onto particular functional networks (e.g., the mapping is relatively close for TDPC pathology with the semantic network, whereas most proteinopathies involve the salience network). The scheme makes specific predictions about the sequence of regional involvement with particular proteinopathies (e.g., sequential involvement of homologous contralateral temporal lobe regions with TDPC pathology) (see also Figure 3, main text).