Abstract
The components of Magnolia officinalis have well known to act anti-inflammatory, anti-oxidative and neuroprotective activities. These efficacies have been sold many products as nutritional supplement extracted from bark of Magnolia officinalis. Thus, to assess and compare neuroprotective effect in the nutritional supplement (Magnolia ExtractTM, Health Freedom Nutrition LLC, USA) and our ethanol extract of Magnolia officinalis (BioLand LTD, Korea), we investigated memorial improving and anti-Alzheimer’s disease effects of extract products of Magnolia officinalis in a transgenic AD mice model. Oral pretreatment of two extract products of Magnolia officinalis (10 mg/kg/day in 0.05% ethanol) into drinking water for 3 months ameliorated memorial dysfunction and prevented Aβ accumulation in the brain of Tg2576 mice. In addition, extract products of Magnolia officinalis also decreased expression of β-site APP cleaving enzyme 1 (BACE1), amyloid precursor protein (APP) and its product, C99. Although both two extract products of Magnolia officinalis could show preventive effect of memorial dysfunction and Aβ accumulation, our ethanol extract of Magnolia officinalis (BioLand LTD, Korea) could be more effective than Magnolia ExtractTM (Health Freedom Nutrition LLC, USA). Therefore, our results showed that extract products of Magnolia officinalis were effective for prevention and treatment of AD through memorial improving and anti-amyloidogenic effects via down-regulating β-secretase activity, and neuroprotective efficacy of Magnolia extracts could be differed by cultivating area and manufacturing methods.
Keywords: Magnolia, Tg2576, Alzheimer’s disease, Natural product
INTRODUCTION
The bark of the root and stem of Magnolia family has been used in oriental traditional medicine to various diseases such as neurosis, anxiety, stroke, fever, gastrointestinal disorders, allergic disease and headache (Watanabe et al., 1983; Song et al., 1989; Kuribara et al., 1998). To define these effects, researchers isolated many bioactive components such as honokiol, obovatol, magnolol and 4-O-methylhonokiol which have anti-inflammatory (Lin et al., 2007; Munroe et al., 2007; Zhou et al., 2008), neuroprotetive (Lin et al., 2006) and anti-oxidative effect (Fujita and Taira, 1994; Chen et al., 2007; Dikalov et al., 2008). We previously reported that 4-O-methylhonokiol have memory improving functions in several different animal models; scopolamine-infused model (Lee et al., 2009), Aβ-infused animal model (Lee et al., 2010; Lee et al., 2011c), systemic LPS-induced animal model (Lee et al., 2012b), and presenilin 2 mutant (Lee et al., 2011a), Swedish APP and Tg2576 AD mouse models (Lee et al., 2012d). In addition, we reported that obovatol also have memory improving effects in systemic LPS-induced animal model (Choi et al., 2012a) and Tg2576 AD mouse models (Choi et al., 2012b). Hou et al., (2000) reported that co-treatment of magnolol and honokiol may enhance in vivo hippocampal acetylcholine release. The co-treatment of magnolol and honokiol was reported to prevent age-related learning and memory impairment in senescence-accelerated-prone 8 (SAMP8) mice (Matsui et al., 2009). In addition, neuroprotective effect in magnolol or honokiol via anti-oxidative effect was also reported by many researchers (Lin et al., 2006; Cui et al., 2007; Hoi et al., 2010). These studies indicate that extract of Magnolia officinalis (ME) could have various neuroprotective effects as well as many health benefit effects. For this reason, several extract products of Magnolia family have been sold as dietary supplement for benefit of health, and used by many people in the worldwide.
Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 50 to 75% of all cases (Blennow et al., 2006; Ferri et al., 2009). AD is pathologically characterized by intraneuronal neurofibrillary tangles and extraneuronal senile plaques, which are primarily composed of aggregates of the microtubule-associated protein tau, and the amyloid beta (Aβ) peptide, respectively (Castellani et al., 2007). Aβ is proteolytically produced from the amyloid precursor protein (APP) by proteases, called β- or γ-secretases (De Strooper and Annaert, 2000). Because the deposition of Aβ contributes to progressive degeneration and loss of neurons in the brain, the procedure of Aβ production and deposition has been critically targeted as therapeutic strategies against AD (Golde et al., 2006). β-Secretase known as β-site APP cleaving enzyme I (BACE1) is considered as a major target for preventing amy-loidogenesis in the therapy and/or prevention of AD (Yan et al., 1999; Lin et al., 2000). BACE1 is upregulated by a variety of risk factors such as hypoxia, mitochondrial dysfunction and oxidative stress (Cole and Vassar, 2007). Thus, inhibitory effect of these risk factors is also considered as targets for pre-vention and treatment of AD. Transgenic models of AD have been made by expressing human APP, presenilin 1 or presenilin 2 gene, and they showed agerelated cognitive impairment, and concurrent deposition of Aβ plaque (Hsiao, 1998; Janus and Westaway, 2001; Duff and Suleman, 2004). The Tg2576 mice overexpress a mutant human Aβ precursor protein and develop impairment of spatial memory and deposition of Aβplaque in cortex and hippocampus (Hsiao et al., 1996; Chap-man et al., 1999; Westerman et al., 2002). Thus, Tg2576 mice have been used to investigate memorial improving and antiamyloidogenic effect of various new developed drugs.
In this study, we investigated and compared the memory improving and anti-amyloidogenic effects of two ME products on the Tg2576 mice. We found that both two ME products pre-vented memory impairment through prevention of amyloido-genesis in the Tg2576 mice.
MATERIALS AND METHODS
Magnolia extract
Our ethanol extract of Magnolia officinalis (BL-ME) was supplied by Bioland Ltd. (Chungnam, Korea) as an ethanol extract of 99.6% purity. The bark of Magnolia officinalis was purchased from Kyung-Dong Market, Seoul, Korea, and taxonomically
identified by Dr. Bang Yeon Hwang at the Research Institute of Drug Resource, Chungbuk National University (Cheongju, Korea). A voucher specimen (CBNU 9301) was deposited at the Herbarium of Chungbuk National University, Chungbuk, Korea. The bark of Magnolia officinalis was dried in the shade at room temperature and stored in a dark, cold room until use. The air-dried bark of Magnolia officinalis (3 kg) was cut into pieces and extracted twice with 95% (v/v) ethanol (four times as much as the weight of the dried plants) for 3 days at room temperature. After filtration through the 400 mesh filter cloth, the filtrate was re-filtered through filter paper (Whatman, No. 5) and concentrated under reduced pressure. The BL-ME contained 1.24% magnolol, 1.81% honokiol, 1.24% 4-O-methylhonokiol and others (unpublished data). Magnolia ExtractTM (HFN-ME) was purchased from Health Freedom Nutrition, LLC (Reno, Nevada, USA), and contained 4.5% of honokiol and magnolol.
In our previous studies, treatment of 1 mg/kg/day 4-O-methylhonokiol showed memory improving and neuroprotective effect in various animal models (Lee et al., 2010; Choi et al., 2011; Lee et al., 2011a; Lee et al., 2011c). Therefore, we used 10 mg/kg/day ME products including these components to administrate toward in vivo animal models in this study. The ME products dissolved in 0.05% ethanol were added to drinking water and mice were allowed access to it for 3 months ad libitum before induction of memory impairment.
Alzheimer’s disease mouse model
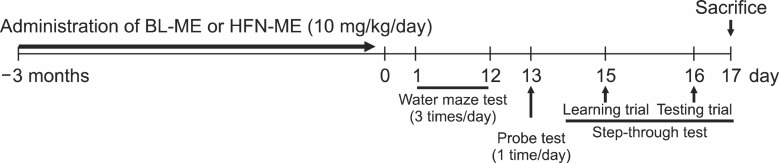
Transgenic lines, overexpressing APP the K670M/N671L mutant gene, were established by backcrossing the founder mice with the parental strain of C57BL/6-SJL mice as described elsewhere (Hsiao et al., 1996). 12-months-old transgenic mice and age-matched normal C57BL/6 mice were purchased from Taconic Farms (Germantown, NY, USA), and were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) of Laboratory Animal Research Center at Chungbuk National University, Korea (CBNU-404-12-01). All mice were housed in a room that was automatically maintained at 21-25℃ and relative humidity (45-65%) with a controlled light-dark cycle. Experimental scheme was shown in Fig. 1.
Fig. 1. Experimental scheme for administering two ME products to Tg2576 mice. Tg2576 mice were treated with BL-ME or HFN-ME (10 mg/kg/day) into drinking water for 3 months. Then, water maze test was performed at 3 times per day for 12 days. A probe test was performed 24 hr after the water maze test. Then, passive avoidance step-through test was 48 hr after the probe test. After step-through test, sacrifice was performed and molecular biological assays were performed. BL-ME: Ethanol extract of Magnolia officinalis from Bioland LTD, HFN-ME: Extract product of Magnolia officinalis from Health Freedom Nutrition LLC.
Water maze test
The water maze test is also a widely accepted method for memory test, and we performed this test as described by Morris (1984). Maze testing was performed by the SMART-CS (Panlab, Barcelona, Spain) program and equipment. A circular plastic pool (height: 35 cm, diameter: 100 cm) was filled with milky water kept at 22-25℃. An escape platform (height: 14.5 cm, diameter: 4.5 cm) was submerged 0.5-1 cm below the surface of the water in position. On training trials, the mice were placed in a pool of water and allowed to remain on the platform for 10 sec and were then returned to the home cage during the second-trial interval. The mice that did not find the platform within 60 sec were placed on the platform for 10 sec at the end of trial. They were allowed to swim until they sought the escape platform. These trials were performed in single platform and in three starting positions of rotational starting. Escape latency, escape distance, swimming speed and swimming pattern of each mouse was monitored by a camera above the center of the pool connected to a SMART-LD pro-gram (Panlab, Barcelona, Spain).
Probe test
A probe trial in order to assess memory consolidation was performed 24 hr after the water maze tests. In this trial, the platform was removed from the tank, and the mice were allowed to swim freely. For these tests, percentage time in the target quadrant and target site crossings within 60 sec was recorded. The time spent in the target quadrant is taken to indicate the degree of memory consolidation that has taken place after learning. Swimming pattern of each mouse was monitored by a camera above the center of the pool connected to a SMART-LD program described above.
Passive avoidance performance test
The passive avoidance test is also widely accepted as a simple and rapid test method for measuring memory capacity. The passive avoidance response was determined using a "step-through" apparatus (Med Associates, Inc., Georgia, VT, USA) that is consisted of an illuminated and a dark compartments (each 20.3×15.9×21.3 cm) adjoining each other through a small gate with a grid floor, 3.175 mm stainless steel rod set 8 mm apart. One day after water maze test, training trial was performed. The mice were placed in the illuminated compartment facing away from the dark compartment. When the mice moved completely into the dark compartment, it received an electric shock (1 mA, 3 sec duration). Then the mice were returned to their home case. At one day later, the mice were placed in the illuminated compartment and the latency period to enter the dark compartment defined as "retention" was measured. The time when the mice entered in the dark compartment were recorded and described as step-through latency. The retention trials were set at a limit of 180 sec of cut-off time.
Brain collection and preservation
After behavioral test (step through test), animals were perfused with saline under ether inhalation. The brains were immediately collected and separated into cortical and hippocampal regions. All the brain regions were immediately stored at-80℃, and used to biological assay.
Immunohistochemistry
Mice were anesthetized with ether. While general anesthesia, the mice received intracardiac perfusion with 20 ml of saline, followed by 50 ml of phosphate-buffered saline (PBS) containing 4% paraformaldehyde. After perfusion, the brain were removed from the skull and post-fixed for 2-4 hr in the same fixative, and were then cryoprotected overnight in 30% sucrose prepared in PBS. Serial coronal sections of brain (40㎛) were cut with a freezing microtome. Immunohistochemical staining was performed using the avidin-biotin peroxidase method. The sections were incubated overnight at 4℃ with anti-Aβ1-42 (1:2000 dilution, Covance, Berlely, CA, USA). After washing in PBS, the sections were incubated in biotinylated goat anti-mouse IgG (1:2000 dilution, Vector Labora-tories, Burlingame, CA, USA) for 1 hr at room temperature. The sections were subsequently washed and incubated with avidin-conjugated peroxidase complex (ABC kit, 1:200 dilution, Vector Laboratories) for 30 min followed by PBS washing. The peroxidase reaction was performed in PBS using 3, 3’-diaminobenzidine tetrahydrochloride (DAB, 0.02%) as the chromogen. Finally, the sections were rinsed, mounted on poly-glycine-coated slides, dehydrated, and cover-slipped for light microscopy and photography.
Western blotting analysis
Tissues were homogenized with lysis buffer [50 mM Tris pH 8.0, 150 mM sodium chloride (NaCl), 0.02% sodium azide,0.2% sodium dodecyl sulfate (SDS), 1 mM phenylmethane-sulphonyl fluoride, 10 μg/ml aprotinin, 1% IGAPEL CA-630, 10 mM sodium fluoride, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM ethylene glycol tetraacetic acid (EGTA) and 0.5% sodium deoxycholate] and centrifuged at 15,000×g for 15 min. Equal amount of proteins (40 μg) were separated on a SDS/10 and 15% polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane (GE Water and Process Technologies). Blots were blocked for 1 hr at room temperature with 5% (w/v) non-fat dried milk in Tris-buffered saline [10 mM Tris (pH 8.0) and 150 mM NaCl] solution containing 0.05% Tween 20. The membrane was then incubated for 3 hr at room temperature with specific antibodies: anti-APP (1:500 dilution, ABR-affinity Bioreagents), anti-BACE1 (1:500 dilution, Sigma) and anti-β-actin (1:2000 dilution, Santa Cruz Biotechnology) were used. The blots were then incubated with the corresponding conjugated anti-rabbit and anti-mouse immunoglobulin G-horseradish peroxidase (Santa Cruz Biotechnology). Immunoreactive proteins were detected with the ECL Western blotting detection system.
Measurement of Aβ1-42
Lysates of brain tissue were obtained through protein extraction buffer containing protease inhibitor. Aβ1-42 levels were determined using specific ELISA Kit (Immuno-Biological Laboratories Co., Ltd., Takasaki-Shi, Gunma, Japan). In brief, 100μl of sample was added into the precoated plate and was incubated for overnight at 4℃. After washing each well of the precoated plate with washing buffer, 100 μl of labeled antibody solution was added and the mixture was incubated for 1 hr at 4℃ in the dark. After washing, chromogen was added and the mixture was incubated for 30 min at room temperature in the dark. Finally, the resulting color was assayed at 450 nm using a microplate absorbance reader (SunriseTM, TECAN, Switzer-land) after adding stop solution.
Statistical analysis
Statistical analysis of the data was carried out using analysis of variance (ANOVA) for repeated measures followed by Dunette’s post-hoc analysis using GraphPad Prism 4 software (Version 4.03, GraphPad software, Inc.).
RESULTS
ME products ameliorated learning and memory impairment in Tg2576 mice of AD model
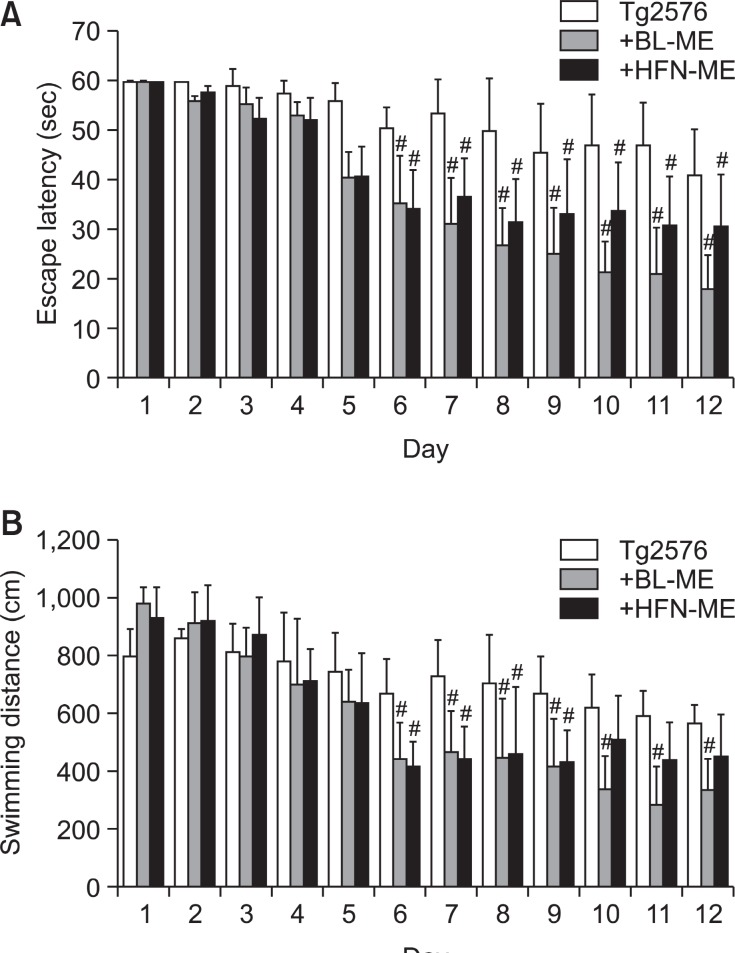
To investigate and compare preventive effect of ME products against memory impairment and Aβ1-42 depositions in the AD model, 12 months old Tg2576 transgenic mice were continuously administrated BL-ME or HFN-ME at a dose of 10 mg/kg/day daily for 3 months, and then compared memory deficiency with the non-treated Tg2576 mice using the water maze test, probe test and step-through test as shown in Fig. 1. The Tg2576 mice were trained for three trials per day during 12 days when most of Tg2576 mice could get maximum memory for escaping platform. Then, the Tg2576 mice were analyzed to locate and escape onto the platform, and their spatial learning scores were recorded. Significant differences by memory improving effect on Tg2576 mice were shown after 5 days, and sufficient learning of platform for probe test was required 12 days. The mice exhibited gradual decreased in escape latency by the training. However, the escape latency of Tg2576 mice was not much reduced than that of ME-treated Tg2576 mice. Oral treatment of all ME products (10 mg/kg/day daily for 3 months) significantly ameliorated memory dysfunc-tion in the AD model mice. Statistical analysis of data from day 6 to day 12 showed the significance of memory improving effect by ME products. Escape latency of the BL-ME treated
group (20.7 ± 9.5 sec, p=0.028) and the HFN-ME treated group (30.6 ± 9.8 sec, p=0.046) on day 11 was shorter than that of the non-treated Tg2576 group (46.7 ± 8.6.0 sec) (Fig.2A). Swimming distance of the BL-ME treated group (280.0± 131.9 cm, p=0.031) and the HFN-ME treated group (438.5± 130.47 cm, p=0.063) on day 11 was shorter compared with that of the non-treated Tg2576 group (588.12 ± 88.18 cm) (Fig. 2B). However, there was no significant difference on average speed between the non-treated group and the two ME-treated groups (data not shown). Although all of ME products showed to ameliorate memorial dysfunction in memorial ability test using water maze test, BL-ME treatment was more effec-tive than the HFN-ME treatment.
Fig. 2. Memory improving effect of two ME products on water maze test in Tg2576 mice. Training trial was performed 3 times per day for 12 days. Escape latency (A) and swimming distance (B) to arrive at platform was automatically recorded in water maze test. Values are presented as mean ± S.E.M. from 10 mice. #Significant difference from non-treated Tg2576 mice (p<0.05). BL-ME: Ethanol extract of Magnolia officinalis from Bioland LTD, HFN-ME: Extract product of Magnolia officinalis from Health Freedom Nutrition LLC.
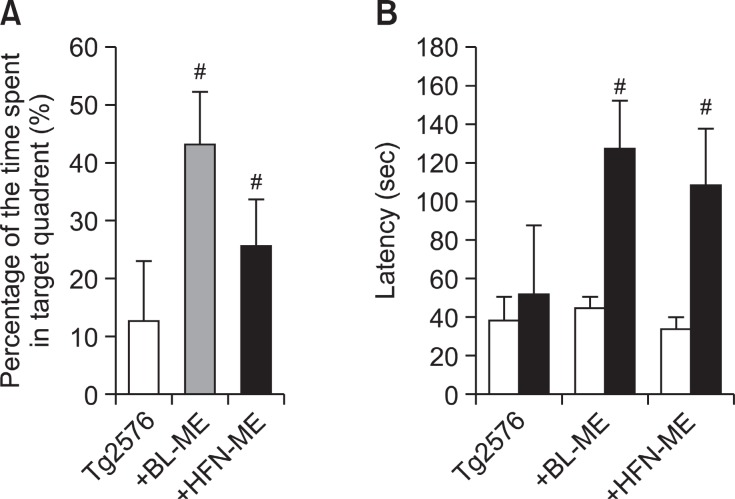
After the water maze test, we performed a probe test to analyze maintenance of memory. During the probe test, the time spent in the target quadrant on the BL-ME treated group (43.1 ± 8.9%, p=0.002) and the HFN-ME treated group (25.6± 8.2%, p=0.043) was significantly increased compared to the non-treated Tg2576 group (12.5 ± 10.4%) (Fig. 3A). Although all of ME products showed to ameliorate memorial dysfunction in memorial ability test using probe test, BL-ME treatment was also more effective than the HFN-ME treatment. We then evaluated learning and memory capacities by the passive avoidance test by step-through method. In the passive avoidance test, there was no significant difference on the learning trial. However, in test trial, step-through latency of the non-treated Tg2576 mice group (51.1 ± 36.6 sec) significantly increased to 114.3 ± 27.8 sec (p=0.005) by BL-ME treatment and 94.9± 31.2 sec (p=0.02) by HFN-ME treatment (Fig. 3B), and effects of two ME products did not show significant difference in step-through test.
Fig. 3. Memory improving effect of two ME products on probe test (A) and step-through test (B) in Tg2576 mice. The time spent in target zone in a probe test (A) conducted following the completion of training was recorded. Values are presented as mean ± S.E.M. from 10 mice. To perform passive avoidance test (B), the mice were given electric shock when entered dark room for training on learning day. After one day, of the learning day the retention time in illuminated compartment was recorded. Values are presented as mean ± S.E.M. from 10 mice. #Significant difference from non-treated Tg2576 mice (p<0.05). BL-ME: Ethanol extract of Magnolia officinalis from Bioland LTD HFN-ME: Extract product of Magnolia officinalis from Health Freedom Nutrition LLC.
ME products prevented Aβ1-42 accumulation in the mice brain of AD model
Many studies reported that Aβ1-42 accumulation, which
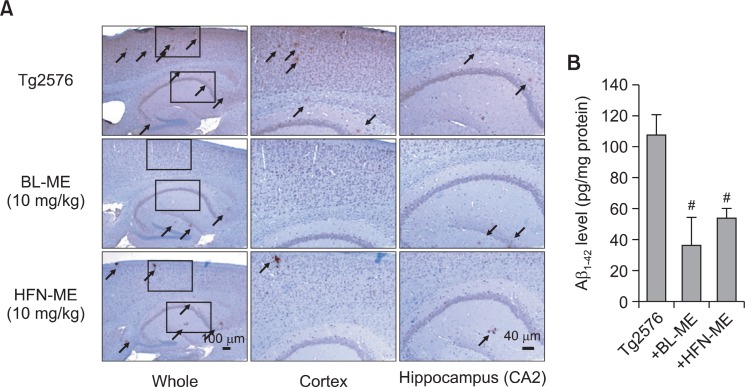
is thought to be a major cause of AD, occurred in the brain of Tg2576 mice. Thus, we investigated and compared anti-amyloidogenic effects of two ME products. The immunohistochemical analysis using Aβ1-42 specific antibody showed Aβ1-42 deposition in the cortical and hippocampal regions of Tg2576 mice brain. However, administration of two ME products significantly decreased accumulated Aβ1-42 plaques in the brain (Fig. 4A). In particular, accumulated Aβ1-42 on brain of BL-ME-treated Tg2576 mice was slightly lower than that of HFN-ME-treated Tg2576 mice. Then, to confirm the decrease of Aβ1-42 plaque by immunohistochemical analysis, we performed quantitative analysis of Aβ1-42 level using ELISA kit. In parallel with the immunohistochemical result, high Aβ1-42 level on brains of Tg2576 mice was detected as previous reported (Lee et al., 2012d), and administration of two ME products
Fig. 4. Inhibitory effect of two ME products on Ab accumulation in the Tg2576 mice brains. Aβ accumulation in the brain was determined using immunohistochemical analysis by Aβ1-42 specific antibody. Elevated accumulation of extracellular Aβ1-42 was observed by immunohistochemical method as described in the materials and methods (A). Arrow indicated the accumulation of Aβ1-42. The effect of two ME products on Aβ1-42 level in the brain was measured by ELISA kit (B). Values are presented as mean ± S.E.M. from 5 mice. #Significant difference from non-treated Tg2576 mice (p<0.05). BL-ME: Ethanol extract of Magnolia officinalis from Bioland LTD, HFN-ME: Extract product of Magnolia officinalis from Health Freedom Nutrition LLC.
significantly decreased these levels (Fig. 4B). In particular, administration of BL-ME slightly more decreased Aβ1-42 levels than that of HFN-ME (Fig. 4B).
ME products inhibited amyloidogenesis in the mice brain of AD model
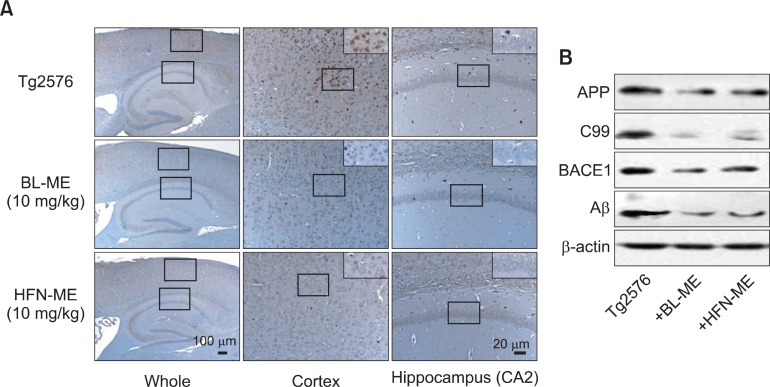
Aβ1-42 is produced from sequential proteolytic cleavage of APP by β- and γ-secretase. β-Secretase is the first step in the production of the Aβ1-42 (Selkoe, 1998). In parallel with the reduced Aβ1-42 level in Tg2576 mice treated with ME products, the number of BACE1 specific antibody-reactive cells were also significantly reduced in cortex and hippocampus of Tg2576 mice treated with ME products (Fig. 5A). To confirm reduction of accumulated Aβ level and BACE1 reactive cells by two ME products, we investigated the expression of full-length APP, BACE1, its processing product C99 and Aβ. Both expression level of full-length APP, C99, BACE1 and Aβincreased in brain of Tg2576 mice, and these levels were reduced by treatment with ME products (Fig. 5B). Although both ME products showed anti-amyloidogenic effects, BL-ME treatment was more effective than the HFN-ME treatment.
Fig. 5. Anti-amyloidogenic effect of two ME products in the brains of Tg2576 mice. Expression of β-secretase were investigated in the cortex and hippocampus regions (A), and expression of APP, C99, BACE1 and Aβ were also detected in the brain (B). BL-ME: Ethanol extract of Magnolia officinalis from Bioland LTD, HFN-ME: Extract product of Magnolia officinalis from Health Freedom Nutrition LLC.
DISCUSSION
The bark of the Magnolia officinalis has been used in traditional herbal medicines in Korea, China and Japan (Iwasaki et al., 2000; Luo et al., 2000). The bark of Magnolia officinalis is known as a rich source of biologically active compounds. It has been reported to have at least 255 different ingredients, such as alkaloids, coumarins, flavonoids, lignans, neolignans, phenylpropanoids and terpenoids (Ito et al., 1982; Tachikawa et al., 2000). The Magnolia bark in the traditional medicines has been known to have anti-cancer effects (Choi et al.,2002), anti-inflammatory effects (Kang et al., 2008) and anti-oxidant actions (Kong et al., 2000). In particular, the Magnolia bark showed anti-stress, anti-anxiety (Weeks, 2009), anti-depressant (Xu et al., 2008), anti-Alzheimer and anti-stroke effects (Lee et al., 2010; Lee et al., 2011b). Recently, we found that oral administration of ethanol extract of Magnolia officina-lis (2.5, 5 or 10 mg/kg) ameliorated the Aβ1-42 (0.5 μg/mouse, i.c.v.)-induced memory impairments and inhibited apoptotic neuronal cell death as well as BACE1 expression (Lee et al., 2010), and also inhibited memory impairments and Aβ1-42 accumulation on the human APP695-expressing Tg2576 mice (Lee et al., 2012c).
In this study, to confirm the preventive effect of ME products and compare neuroprotective effect of two ME products on memory impairment of human APP695-expressing Tg2576 mice, we respectively administrated previous maximum concentration (10 mg/kg/day) of two ME products for 3 months, and found the memory improving effect of all ME products in Tg2576 mice of AD model through behavior tests such as water maze test, probe test and step-through test. Although all ME products were effective for preventing memory impairment, escape latency of the BL-ME treated group on day 11 of water maze test was twice shorter than that of HFN-ME, and the time spent on probe test was also twice longer than that of HFN-ME (Fig. 2A and 3A). Thus, memory improving effect of BL-ME was slightly higher than that of HFN-ME. Administration of two ME products inhibited Aβ1-42 production and accumulation in Tg2576 mice. In particular, Aβ1-42 level in BL-ME treated group was about 60% lower than that of untreated group and about 20% lower than that of HFN-ME (Fig. 4B), indicating that anti-amyloidogenic effect of BL-ME was slightly effective compare to that of HFN-ME. These differences were believed to be contributed by ratio of components or producing district of Magnolia trees as previously reported (Lee et al., 2011b). These results suggest that all ME products could prevent occurrence and progress of AD.
The ME and its components such as magnolol, honokiol, obovatol and 4-O-methyohonokiol have been also reported to have neuroprotective effect through several mechanisms. Co-treatment of magnolol and honokiol was reported to enhance hippocampal acetylcholine release (Hou et al., 2000). It was reported that co-treatment of magnolol and honokiol in SAMP8 mice prevented age-related learning and memory impairment (Matsui et al., 2009). The neuroprotective effect of magnolol or honokiol was reported to occur via its anti-oxidative effect (Lin et al., 2006; Cui et al., 2007; Hoi et al., 2010). Lee et al., (2012a) reported that obovatol has neuroprotective and anti-oxidative effect in SH-SY5Y neuroblastoma cells. Recently, we also found that obovatol has neuroprotective effect in human APP-overexpressed Tg2576 model and LPS-induced AD animal model (Choi et al., 2012a). We reported that 4-O-methylhonokiol has memory improving and neuroprotective functions in several different animal models such as scopolamine-infused model (Lee et al., 2009), LPS-induced AD model (Lee et al., 2012b), Aβ-infused model (Lee et al., 2010; Lee et al., 2011c), presenilin 2 mutant (Lee et al., 2011b), Swedish APP (APPsw) AD mouse models (Choi et al., 2011), and human APP overexpressed (Tg2576) AD mouse mocels (Lee et al., 2012d). We found several functional mechanisms such as inhibition of Aβ1-42-induced ROS generation and neuronal cell death (Lee et al., 2010), inactivation of β-secretase and ERK pathway (Lee et al., 2011a) as well as inhibition of cytokine release and NF-κB activity by 4-O-methylhonokiol in different animal models (Lee et al., 2012b). Thus, many studies reported anti-amyloidogenic effect of many components of ME through its multiple mechanisms, suggesting that crude extract of Magnolia officinalis including these components can also show memory improving and neuroprotective functions in AD patient as well as elderly hu-man. In particular, it is possible that synergistic effect in these components induce more effective neuroprotective functions as well as memory improving effects. For this reason, several extract products of Magnolia family have been sold as dietary supplement by several companies.
Although pharmacodynamics and pharmacokinetics of whole components of Magnolia extract were not investigated yet, pharmacodynamics and pharmacokinetics of single component was reported by several investigators. (Lin et al., 2011) reported that the magnolol was predominant in the liver, kidney, brain, lung and heart after intravenous (i.v.) or oral administration to Sprague-Dawley rats. Ten minutes after magnolol (5 mg/kg, i.v.) administration to rat, brain concentrations of magnolol showed no significant differences among various re-gions (cerebral cortex, olfactory bulb, hippocampus, striatum, cerebellum, brain stem and rest of brain) (Tsai et al., 1996). We found that orally administered 4-O-methylhonokiol in ICR mice rapidly disappears from the blood and is distributed into brain rapidly (less than 1 hr after treatment). In addition, we found that the 4-O-methylhonokiol could be accumulated into brain, and about 50-100 ng/ml may be effective dose in the brain (unpublished data). Pharmacokinetic profile in Tg2576 mice supposes to be similar with ICR mice. Thus, we can assume that the similar results could be obtained in Tg2576 mice. Further, we will investigate bioavailability and pharmacokinetic studies in the Tg2576 mice. In addition, several ME products are recommended to be taken as 1.5-5 mg/kg/daily from each company such as Health Freedom Nutrition LLC (Reno, Nevada, USA), Allergy Research Group LLC (Alameda, California, USA), and HealthAid Ltd (London, UK). Thus, we believe that dosage of 10 mg/kg/day is appropriate for analyzing memory improving and anti-amyloidogenic effects of two ME products.
In summary, two ME products ameliorated memory impairment in Tg2576 mice, and effectively reduced Aβ accumulation via BACE1 expression. In addition, memory improving and anti-amyloidogenic effect of BL-ME was slightly more effective compared to that of HFN-ME. These data suggest that ME is a strong potential candidate for treatment of AD.
Acknowledgments
This work was supported by the National Research Foundation of Korea [NRF] grant funded by the Korea government [MEST] (MRC, 2010-0029480), and by a grant (No. A101836) of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, and by Priority Research Centers Program through the National Research Foundation of Korea (NRF) founded by the Ministry of Education, Science and Technology (2011-0031403).
References
- 1.Blennow K. de Leon M. J. Zetterberg H. Alzheimer's disease. Lancet. (2006);368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Castellani R. J. Zhu X. Lee H. G. Moreira P. I. Perry G. Smith M. A. Neuropathology and treatment of Alzheimer disease: did we lose the forest for the trees? Expert Rev. Neu-rother. (2007);7:473–485. doi: 10.1586/14737175.7.5.473. [DOI] [PubMed] [Google Scholar]
- 3.Chapman P. F. White G. L. Jones M. W. Cooper-Blacketer D. Marshall V. J. Irizarry M. Younkin L. Good M. A. Bliss T. V. Hyman B. T. Younkin S. G. Hsiao K. K. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. (1999);2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 4.Chen C. L. Chang P. L. Lee S. S. Peng F. C. Kuo C. H. Chang H. T. Analysis of magnolol and honokiol in biological fluids by capillary zone electrophoresis. J. Chromatogr. A. (2007);1142:240–244. doi: 10.1016/j.chroma.2006.12.099. [DOI] [PubMed] [Google Scholar]
- 5.Choi D. Y. Lee J. W. Lin G. Lee Y. K. Lee Y. H. Choi I. S. Han S. B. Jung J. K. Kim Y. H. Kim K. H. Oh K. W. Hong J. T. Lee M. S. Obovatol attenuates LPS-induced memory impairments in mice via inhibition of NF-κB signaling pathway. Neu-rochem.Int. (2012a);60:68–77. doi: 10.1016/j.neuint.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Choi D. Y. Lee J. W. Peng J. Lee Y. J. Han J. Y. Lee Y. H. Choi I. S. Han S. B. Jung J. K. Lee W. S. Lee S. H. Kwon B. M. Oh K. W. Hong J. T. Obovatol improves cognitive functions in animal models for Alzheimer's disease. J. Neurochem. (2012b);120:1048–1059. doi: 10.1111/j.1471-4159.2011.07642.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi I. S. Lee Y. J. Choi D. Y. Lee Y. K. Lee Y. H. Kim K. H. Kim Y. H. Jeon Y. H. Kim E. H. Han S. B. Jung J. K. Yun Y. P. Oh K. W. Hwang D. Y. Hong J. T. 4-O-methylhonokiol attenuated memory impairment through modulation of oxidative damage of enzymes involving amyloid-β generation and accumu-lation in a mouse model of Alzheimer's disease. J. Alzheimers Dis. (2011);27:127–141. doi: 10.3233/JAD-2011-110545. [DOI] [PubMed] [Google Scholar]
- 8.Choi J. H. Ha J. Park J. H. Lee J. Y. Lee Y. S. Park H. J. Choi J. W. Masuda Y. Nakaya K. Lee K. T. Costunolide triggers apoptosis in human leukemia U937 cells by depleting intracellular thiols. Jpn. J. Cancer Res. (2002);93:1327–1333. doi: 10.1111/j.1349-7006.2002.tb01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole S. L. Vassar R. The Alzheimer's disease beta-secretase enzyme BACE1. Mol. Neurodegener. (2007);2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui H. S. Huang L. S. Sok D. E. Shin J. Kwon B. M. Youn U. J. Bae K. Protective action of honokiol administered oral-ly against oxidative stress in brain of mice challenged with NMDA. Phytomedicine. (2007);14:696–700. doi: 10.1016/j.phymed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 11.De Strooper B. Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. (2000);113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 12.Dikalov S. Losik T. Arbiser J. L. Honokiol is a potent scavenger of superoxide and peroxyl radicals. Biochem. Pharma-col. (2008);76:589–596. doi: 10.1016/j.bcp.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duff K. Suleman F. Transgenic mouse models of Alzheimer's disease: how useful have they been for therapeutic development? Brief. Funct. Genomic. Proteomic. (2004);3:47–59. doi: 10.1093/bfgp/3.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Prince M, editor; Jackson J, editor. World Alzheimer Report 2009 - Executive Summary. Alzheimer’s Disease Inter-national; London.: pp. 1–22. [Google Scholar]
- 15.Fujita S. Taira J. Biphenyl compounds are hydroxyl radi-cal scavengers: their effective inhibition for UV-induced mutation in Salmonella typhimurium TA102. Free Radic. Biol. Med. (1994);17:273–277. doi: 10.1016/0891-5849(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 16.Golde T. E. Dickson D. Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer's disease. Curr. Alzheimer Res. (2006);3:421–430. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 17.Hoi C. P. Ho Y. P. Baum L. Chow A. H. Neuroprotective effect of honokiol and magnolol compounds from Magnolia officinalis on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. (2010);24:1538–1542. doi: 10.1002/ptr.3178. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y. C. Chao P. D. Chen S. Y. Honokiol and magnolol increased hippocampal acetylcholine release in freely-moving rats. Am. J. Chin. Med. (2000);28:379–384. doi: 10.1142/S0192415X00000441. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao K. Transgenic mice expressing Alzheimer amyloid precursor proteins. Exp. Gerontol. (1998);33:883–889. doi: 10.1016/s0531-5565(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao K. Chapman P. Nilsen S. Eckman C. Harigaya Y. Younkin S. Yang F. Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. (1996);274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 21.Ito K. Iida T. Ichino K. Tsunezuka M. Hattori M. Namba T. Obovatol and obovatal novel biphenyl ether lignans from the leaves of Magnolia obovata Thunb. Chem. Pharm. Bull.(Tokyo) (1982);30:3347–3353. doi: 10.1248/cpb.30.3347. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki K. Wang Q. Seki H. Satoh K. Takeda A. Arai H. Sasaki H. The effects of the traditional chinese medicine"Banxia Houpo Tang (Hange-Koboku To)" on the swallowing reflex in Parkinson's disease. Phytomedicine. (2000);7:259–263. doi: 10.1016/S0944-7113(00)80042-2. [DOI] [PubMed] [Google Scholar]
- 23.Janus C. Westaway D. Transgenic mouse models of Al-zheimer's disease. Physiol. Behav. (2001);73:873–886. doi: 10.1016/s0031-9384(01)00524-8. [DOI] [PubMed] [Google Scholar]
- 24.Kang J. S. Lee K. H. Han M. H. Lee H. Ahn J. M. Han S. B. Han G. Lee K. Park S. K. Kim H. M. Antiinflamma-tory activity of methanol extract isolated from stem bark of Magnolia kobus. Phytother. Res. (2008);22:883–888. doi: 10.1002/ptr.2386. [DOI] [PubMed] [Google Scholar]
- 25.Kong C. W. Tsai K. Chin J. H. Chan W. L. Hong C. Y. Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock. (2000);13:24–28. doi: 10.1097/00024382-200013010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Kuribara H. Stavinoha W. B. Maruyama Y. Behavioural pharmacological characteristics of honokiol an anxiolytic agent present in extracts of Magnolia bark, evaluated by an elevated plus-maze test in mice. J. Pharm. Pharmacol. (1998);50:819–826. doi: 10.1111/j.2042-7158.1998.tb07146.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee J. W. Lee Y. K. Lee B. J. Nam S. Y. Lee S. I. Kim Y. H. Kim K. H. Oh K. W. Hong J. T. Inhibitory effect of ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on memory impairment and neuronal toxicity induced by beta-amyloid. Phar-macol. Biochem. Behav. (2010);95:31–40. doi: 10.1016/j.pbb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Lee M. Kwon B. M. Suk K. McGeer E. McGeer P. L. Effects of obovatol on GSH depleted glia-mediated neurotoxicity and oxidative damage. J. Neuroimmune. Pharmacol. (2012a);7:173–186. doi: 10.1007/s11481-011-9300-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y. J. Choi D. Y. Choi I. S. Kim K. H. Kim Y. H. Kim H. M. Lee K. Cho W. G. Jung J. K. Han S. B. Han J. Y. Nam S. Y. Yun Y. W. Jeong J. H. Oh K. W. Hong J. T. Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J. Neuroinflammation. (2012b);9:35. doi: 10.1186/1742-2094-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y. J. Choi D. Y. Han S. B. Kim Y. H. Kim K. H. Hwang B. Y. Kang J. K. Lee B. J. Oh K. W. Hong J. T. Inhibitory Effect of Ethanol Extract of Magnolia officinalis on Memory Impairment and Amyloidogenesis in a Transgenic Mouse Model of Alzheimer's Disease via Regulating β-Secretase Activity. Phytother. Res. (2012c) doi: 10.1002/ptr.4643. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Lee Y. J. Choi D. Y. Lee Y. K. Lee Y. M. Han S. B. Kim Y. H. Kim K. H. Nam S. Y. Lee B. J. Kang J. K. Yun Y. W. Oh K. W. Hong J. T. 4-O-methylhonokiol prevents memory impairment in the Tg2576 transgenic mice model of Alzheimer's disease via regulation of β-secretase activity. J. Alzheimers Dis. (2012d);29:677–690. doi: 10.3233/JAD-2012-111835. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y. J. Choi I. S. Park M. H. Lee Y. M. Song J. K. Kim Y. H. Kim K. H. Hwang D. Y. Jeong J. H. Yun Y. P. Oh K. W. Jung J. K. Han S. B. Hong J. T. 4-O-Methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway. Free Radic. Biol. Med. (2011a);50:66–77. doi: 10.1016/j.freeradbiomed.2010.10.698. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y. J. Lee Y. M. Lee C. K. Jung J. K. Han S. B. Hong J. T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. (2011b);130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y. K. Choi I. S. Ban J. O. Lee H. J. Lee U. S. Han S. B. Jung J. K. Kim Y. H. Kim K. H. Oh K. W. Hong J. T. 4-O-methylhonokiol attenuated β-amyloid-induced memory impairment through reduction of oxidative damages via inactivation of p38 MAP kinase. J. Nutr. Biochem. (2011c);22:476–486. doi: 10.1016/j.jnutbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y. K. Yuk D. Y. Kim T. I. Kim Y. H. Kim K. T. Kim K. H. Lee B. J. Nam S. Y. Hong J. T. Protective effect of the ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on scopolamine-induced memory impairment and the inhibition of acetylcholinesterase activity. J. Nat. Med. (2009);63:274–282. doi: 10.1007/s11418-009-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin S. P. Tsai S. Y. Lee Chao P. D. Chen Y. C. Hou Y. C. Pharmacokinetics, bioavailability, and tissue distribution of magnolol following single and repeated dosing of magnolol to rats. Planta Med. (2011);77:1800–1805. doi: 10.1055/s-0030-1271159. [DOI] [PubMed] [Google Scholar]
- 37.Lin X. Koelsch G. Wu S. Downs D. Dashti A. Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. USA. (2000);97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y. R. Chen H. H. Ko C. H. Chan M. H. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. (2007);81:1071–1078. doi: 10.1016/j.lfs.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y. R. Chen H. H. Ko C. H. Chan M. H. Neuropro-tective activity of honokiol and magnolol in cerebellar granule cell damage. Eur. J. Pharmacol. (2006);537:64–69. doi: 10.1016/j.ejphar.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Luo L. Nong Wang J. Kong L. D. Jiang Q. G. Tan R. X. Antidepressant effects of Banxia Houpu decoction a traditional Chinese medicinal empirical formula. J. Ethnopharmacol. (2000);73:277–281. doi: 10.1016/s0378-8741(00)00242-7. [DOI] [PubMed] [Google Scholar]
- 41.Matsui N. Takahashi K. Takeichi M. Kuroshita T. Noguchi K. Yamazaki K. Tagashira H. Tsutsui K. Okada H. Kido Y. Yasui Y. Fukuishi N. Fukuyama Y. Akagi M. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res. (2009);1305:108–117. doi: 10.1016/j.brainres.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 42.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. (1984);11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 43.Munroe M. E. Arbiser J. L. Bishop G. A. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J. Immunol. (2007);179:753–763. doi: 10.4049/jimmunol.179.2.753. [DOI] [PubMed] [Google Scholar]
- 44.Selkoe D. J. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. (1998);8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 45.Song W. Z. Cui J. F. Zhang G. D. Studies on the medicinal plants of Magnoliaceae tu-hou-po of Manglietia. Yao. Xue. Xue. Bao. (1989);24:295–299. [PubMed] [Google Scholar]
- 46.Tachikawa E. Takahashi M. Kashimoto T. Effects of extract and ingredients isolated from Magnolia obovata thunberg on catecholamine secretion from bovine adrenal chromaffin cells. Biochem. Pharmacol. (2000);60:433–440. doi: 10.1016/s0006-2952(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 47.Tsai T. H. Chou C. J. Chen C. F. Pharmacokinetics and brain distribution of magnolol in the rat after intravenous bolus injection. J. Pharm. Pharmacol. (1996);48:57–59. doi: 10.1111/j.2042-7158.1996.tb05877.x. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe K. Watanabe H. Goto Y. Yamaguchi M. Yamamoto N. Hagino K. Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: central depressant effects. Planta Med. (1983);49:103–108. doi: 10.1055/s-2007-969825. [DOI] [PubMed] [Google Scholar]
- 49.Weeks B. S. Formulations of dietary supplements and herbal extracts for relaxation and anxiolytic action: Relarian. Med. Sci. Monit. (2009);15:RA256–262. [PubMed] [Google Scholar]
- 50.Westerman M. A. Cooper-Blacketer D. Mariash A. Kotilinek L. Kawarabayashi T. Younkin L. H. Carlson G. A. Younkin S. G. Ashe K. H. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. (2002);22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Q. Yi L. T. Pan Y. Wang X. Li Y. C. Li J. M. Wang C. P. Kong L. D. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog. Neuropsychopharmacol. Biol. Psychiatry. (2008);32:715–725. doi: 10.1016/j.pnpbp.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Yan R. Bienkowski M. J. Shuck M. E. Miao H. Tory M. C. Pauley A. M. Brashier J. R. Stratman N. C. Mathews W. R. Buhl A. E. Carter D. B. Tomasselli A. G. Parodi L. A. Heinrikson R. L. Gurney M. E. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. (1999);402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 53.Zhou H. Y. Shin E. M. Guo L. Y. Youn U. J. Bae K. Kang S. S. Zou L. B. Kim Y. S. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB JNK and p38 MAPK inactivation. Eur. J. Pharmacol. (2008);586:340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]