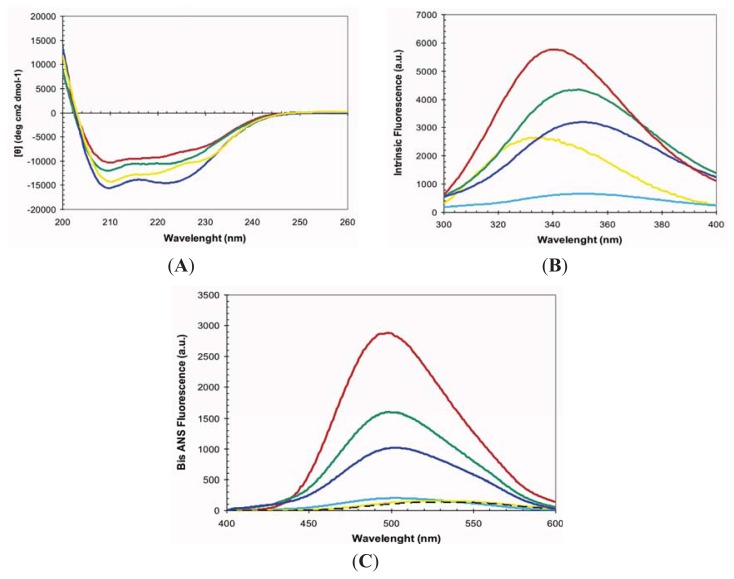
Figure 2.
2,2,2-trifluoroethanol (TFE) dependence of URN1-FF molten globule (MG) conformational properties. Protein samples were prepared at 20 μM and pH 2.5 and immediately measured by (A) far-UV CD, (B) tryptophan intrinsic fluorescence and (C) 4,4′-Dianilino-1,1′-Binaphthyl-5,5′-Disulfonic Acid (bis-ANS) fluorescence at 25 °C. The fluorescence emission spectrum of bis-ANS in the absence of protein is represented as a dotted line. URN1-FF species were measured at 0% (red), 15% (green) and 25% (blue) of TFE (v/v). The spectra of the native protein at pH 5.7 are shown in yellow. In (B) and (C) the spectrum of heat denatured URN1-FF at 90 °C is shown in light blue.