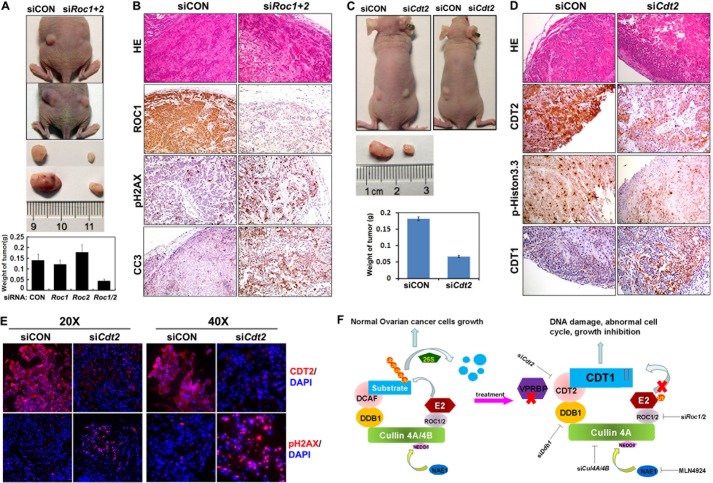
FIGURE 7.
RNAi depletion of Roc1/2 and Cdt2 inhibits ovarian cancer cell growth and survival in vivo. A, OV2008 ovarian cancer cells were transfected with siCON or siRoc1/2 (106 cells for each) and then implanted subcutaneously into the left and right flanks of nude mice, respectively. After 30 days, tumors were excised and weighed (n = 6). B, immunohistochemistry results for ROC1, cleaved caspase 3 (CC3), and pH2AX in tumor tissues without or with Roc1/2 depletion. C, OV2008 ovarian cancer cells were transfected with siCON or siCdt2 (106 cells for each) and then implanted subcutaneously into the left and right flanks of nude mice, respectively. After 30 days, tumors were excised and weighed (n = 6). D, immunohistochemistry results for CDT2, CDT1, and pH2AX in tumor tissues without or with Cdt2 depletion. E, immunofluorescent signals for CDT2 and pH2AX in tumor tissues without or with Cdt2 depletion. F, schematic diagram showing CRL4 function in ovarian cancer cells. Under normal circumstances, a CRL4 E3 complex catalyzes ubiquitin transfer from E2 to protein substrates, which results in their polyubiquitination and targeted degradation by the 26 S proteasome. Thus, CRL4 activity maintains ovarian cancer cell growth. When a CRL4DCAF2 E3 complex is inactivated by RNAi depletion of its essential components, including Roc1/2, Cul4a/4b, Ddb1, or Cdt2, or by pharmaceutical inhibition of cullin neddylation with MLN4924, its substrates CDT1 and others accumulate, which suppresses cancer cell growth and triggers ovarian cancer cell killing pathways, including apoptosis, DNA damage, and G2-M arrest.