Abstract
Alveolar macrophages (AMs) constitute the first line of defence in the lung of all species, playing a crucial role in the regulation of immune responses to inhaled pathogens. A detailed understanding of the function and phenotype of AMs is a necessary pre-requisite to both elucidating their role in preventing opportunistic bacterial colonisation of the lower respiratory tract and developing appropriate preventative strategies. The purpose of the study was to characterise this important innate immune cell at the tissue level by making functional and phenotypic comparisons with peritoneal macrophages (PMs). We hypothesised that the tissue of origin determines a unique phenotype of AMs, which may constitute an appropriate therapeutic target for certain equine respiratory diseases. Macrophages isolated from the lung and the peritoneal cavity of 9 horses were stimulated with various toll like receptor (TLR) ligands and the production of nitrite, tumour necrosis factor alpha (TNFα), interleukin (IL) 10 and indoleamine 2,3-dioxygenase (IDO) were measured by the Griess reaction and enzyme linked immunosorbent assay (ELISA) and/or quantitative polymerase chain reaction, respectively. Cells were also compared on the basis of phagocytic-capacity and the expression of several cell surface markers. AMs, but not PMs, demonstrated increased TNFα release following stimulation with LPS, polyinosinic polycytidylic acid (Poly IC) and heat-killed Salmonella typhinurium and increased TNFα and IDO mRNA expression when stimulated with LPS. AMs showed high expression of the specific macrophage markers cluster of differentiation (CD) 14, CD163 and TLR4, whereas PMs showed high expression of TLR4 only. AMs, but not PMs, demonstrated efficient phagocytic activity. Our results demonstrate that AMs are more active than PMs when stimulated with various pro-inflammatory ligands, thus supporting the importance of the local microenvironment in the activation status of the macrophage. This information provides a valuable knowledge base on which to improve our understanding of the role of macrophages and their microenvironment in equine innate immunity.
Abbreviations: AMs, alveolar macrophages; BALF, bronchoalveolar lavage fluid; CD, cluster of differentiation; cDNA, complementary DNA; FS, forward scatter; HS, horse serum; IAD, inflammatory airway disease; MD2, myeloid differentiation factor 2; NO, nitric oxide; PL, peritoneal lavage; PMs, peritoneal macrophages; Poly IC, polyinosinic polycytidylic acid; qPCR, quantitative PCR; RIN, RNA integrity number; RPMI, Roswell Park Memorial Institute; SC, side scatter; TLR, toll-like receptor
Keywords: Macrophage, Horse, Lungs, Peritoneal cavity, Immunity
1. Introduction
Opportunistic respiratory infections, often with upper airway commensal bacteria, are a recognised cause of lower respiratory tract disease in the horse. Such infections can result in a variety of diseases of varying severity, ranging from subtle athletic performance limitation to life-threatening fulminant pleuropneumonia (Wlaschitz and Scherzer, 2000; Robinson and Hoffman, 2003; Wood et al., 2005; Ferrucci et al., 2008). Although specific risk factors associated with such infections have been well documented (Robinson and Hoffman, 2003; Cardwell, 2009), there remains a lack of understanding of the precise mechanisms which permit colonisation of the lower respiratory tract by otherwise non-pathogenic bacteria, although this is likely to be partly attributable to either suppression of the innate immune response and/or an “overwhelming” of its capabilities.
As a component of the mononuclear phagocyte system (MPS), alveolar macrophages play a pivotal role in the innate immune response of the lung. The MPS comprises resident innate immune cells found in large numbers in every tissue of the body, especially at portals of pathogen entry such as the lungs or the intestines. From their progenitor in the bone-marrow, cells differentiate into monocytes under the influence of hematopoietic factors such as the macrophage CSF (M-CSF or CSF-1), following which they are recruited to various different tissues (Hume et al., 2002; Hume, 2008), which they populate as resident macrophages. At these resident sites, they recognise non self-components of potential pathogens, so-called pathogen-associated molecular patterns (PAMPs), a process mediated through families of pattern recognition receptors on the cell surface, within the endocytic pathways and in the cytoplasm (Kumar et al., 2011), resulting in the production of inflammatory molecules and antimicrobial effectors (Hume et al., 2002). Despite their identical lineage, work in other species has identified a unique phenotype of the alveolar macrophage, compared with macrophages derived from other anatomical sites, a finding thought to reflect the distinctive microenvironment of this cell and its “breath-by-breath” exposure to ambient air containing a variety of potential allergenic and pro-inflammatory components (Guth et al., 2009). In order to further elucidate the potential role of macrophage suppression in various opportunistic bacterial airway diseases in the horse, we considered it essential to further characterise its basic function and phenotype by making specific comparisons with other tissue derived macrophages. The peritoneal cavity constitutes an abundant source of macrophages for such comparative purposes. Furthermore, although peritoneal macrophages have been studied in great detail in mice (Zhang and Morrison, 1993; Katakami et al., 1988; Sherry et al., 2007) and to a lesser extent in humans (Hetland and Bungum, 1988; Halme, 1989), there have been relatively few studies of the functions of peritoneal macrophage populations of the horse (Morris et al., 1992; Hawkins et al., 1998; Laan et al., 2005, 2006). Therefore novel phenotypic and functional data was obtained from macrophages harvested from the peritoneal cavity and airways of horses and appropriate comparisons made.
2. Materials and methods
2.1. Animals
Nine horses (median age: 16 yrs, range: 4–22) were admitted to the Equine Hospital at the Royal (Dick) School of Veterinary Studies for elective euthanasia. Horses were euthanased by the i.v. administration of cinchocaine hydrochloride (25 mg/ml) and secobarbital sodium (400 mg/ml) (Somulose™, Arnolds/Dechra, UK), via a pre-placed 14 gauge i.v. jugular catheter. All of the animals were systemically healthy, none had a chronic or immediate history of respiratory disease and post-mortem examination confirmed the absence of gross lung pathology in all 9 horses. The Veterinary Ethical Review Committee of the School of Veterinary Medicine, University of Edinburgh approved all the protocols involving the use of post-mortem material from these animals.
2.2. Isolation of alveolar (AMs) and peritoneal macrophages (PMs)
Following euthanasia, the trachea was exposed, transected and occluded proximally to prevent blood contamination of the lungs. The thorax was opened and the lungs, with trachea attached, were removed and transferred to a clean working area. The trachea was further transected at a level approximately 15 cm from the carina. Two litres of sterile PBS (D1408, Sigma–Aldrich, USA) were instilled into the lungs, either directly into the trachea or via a cuffed endotracheal tube. Following gentle massage of the lungs, the bronchoalveolar lavage fluid (BALF) was retrieved by gravity following elevation of the lungs above the level of the distal trachea.
For peritoneal lavage, an incision was made in an aseptically prepared area of the ventral abdominal midline down to the level of the linea alba, through which a catheter and trocar were inserted into the peritoneal cavity. Following removal of the trocar, 6 litres of sterile PBS was infused into the peritoneal cavity by gravity. Following abdominal ballottement, the horse was hoisted by the pelvic limbs and the peritoneal cavity opened carefully. The instilled fluid, which had gravitated to a level immediately caudal to the liver, was visualised and retrieved by siphoning through a 1 m length of sterile tubing.
For both the lung and peritoneal lavage, the retrieved fluid was immediately placed on ice and processed within 30 min of collection. Cells were processed as previously described (Laan et al., 2005). Briefly, the fluid was filtered (100 μm cell strainer) and decanted into 500 ml falcon tubes and centrifuged at 400 × g for 10 min at 10 °C. Supernatant was removed carefully and the cell pellets re-suspended in PBS and transferred to a 50 ml falcon tube which was further centrifuged at 400 × g for 5 min at 10 °C. The cell pellet was re-suspended in 1 ml of RPMI-1640 (Sigma–Aldrich, UK) and manually counted using a haemocytometer. Viability was assessed by adding Trypan blue 0.4%. When grossly visible blood contamination was evident, red cells lysis buffer was added for 5 min (10 mM KHCO3, 150 mM NH4Cl, 0.1 mM EDTA pH 8.0) followed by the addition of PBS.
An aliquot was retained for cytological analysis as described previously (Miyamoto et al., 2003). Briefly, cell numbers were adjusted to 4–5 × 105 cells/ml by the addition of a calculated volume of PBS. From this aliquot, 2 cytospin slide preparations were made per lavage (cytospined at 300 rpm for 3 min) and stained (Leishman stain; L/1815L/PB05, Fisher Scientific, Leicestershire, UK) and a differential cell count calculated under light microscopy by counting 500 cells (Hoffman, 1999). Cells were cryopreserved in FCS 90%, DMSO 10% (Sigma–Aldrich, USA) and kept frozen for later use.
2.3. Cell culture
Cells were seeded in 6 well plastic plates (Nunc, Thermo Scientific, Wilmington, USA) at 1 × 106 cells/ml in complete medium: RPMI-1640 medium supplemented with GlutaMAX™-I Supplement (Invitrogen Ltd., Paisley, UK), penicillin/streptomycin (Invitrogen Ltd., Paisley, UK) and 10% heat-inactivated Horse Serum (HS – Sigma Aldrich, cat no: H1138). Plates were incubated at 37 °C and 5% CO2 overnight. The following day, non-adherent cells were removed. New complete medium was added and adherent cells were stimulated with different agonists: LPS (10 ng/ml and 100 ng/ml) from Salmonella enterica serotype Minnesota Re 595 (L9764, Sigma–Aldrich, USA), heat killed Salmonella typhinurium (106 cfu/ml, 107 cfu/ml and 108 cfu/ml) and Poly IC-LMW (Invitrogen, San Diego, USA) (2 μg/ml). Supernatant from the plates was collected at various time-points (0, 2, 6 and 24 h) following stimulation.
2.4. TNFα, IL10 and nitric NO assay
Equine TNFα and IL10 protein production in cell culture supernatants was measured using the DuoSet ELISA kit (R&D systems, Minneapolis, MN) according to the manufacturer's instructions. NO is unstable and it is rapidly converted to nitrite. Thus, nitrite was measured as the end product of NO by Griess reaction as previously described (Gross and Levi, 1992). Positive and negative controls were obtained by using RAW-274.1 cells treated with LPS (10 and 100 ng/ml) for 6 h. OD was read on a Multiskan v2.6 (Thermo Scientific, Wilmington, USA) at 540 nm and data was analysed using Ascent software. Inclusion criteria for the macrophage characterisation were their ability to adhere to the plastic plates for the cytokine and (nitric oxide) NO analysis (Grünig et al., 1991; Hawkins et al., 1998; Moore et al., 2003).
2.5. RNA extraction and quantitative PCR (qPCR)
Following the removal of the cell supernatant at 0 and 6 h post stimulation, total RNA was extracted using RNA-Bee (Amsbio, Abingdon, UK) according to the manufacturer's instructions from both AMs and PMs of 6 and 4 different horses, respectively. RNA concentration and purity was measured using ND-1000 Nanodrop (Thermo Scientific, Wilmington, USA). RNA integrity was also confirmed with RNA 6000 Pico Assay (Agilent Technologies). RNA integrity number (RIN) greater than 7 was considered appropriate for qPCR use. One microgram total RNA was converted to complementary DNA (cDNA) using the prescription NanoScript reverse transcription kit (RT – NanoScript, Primer Design), according to the manufacturer's instruction. cDNA was stored at −20 °C until further use.
In order to select our reference gene, cDNA of the samples (4 LPS treated and 4 untreated) was diluted in RNA/DNA free water to a concentration of 5 ng/ml and 5 μl of each was added to Light Cycler 480 SYBR Green I master mix (Roche). The establishment of the optimal reference genes was performed by using the geNorm™ reference gene selection kit (Primerdesign), according to manufactures instructions. Primers for TNFα and indoleamine 2,3-dioxygenase (IDO) were also provided by Primerdesign. Sense and anti-sense primer sequence for TNFα were ATCTACTCCCAGGTCCTCTTC and CGTGTTGGCAAGGCTCTT, respectively and for IDO ATCAAAGAAATTCCGTTATATTCAA and TGCGTAGACAAGAAGAAGTTATATCAAT, respectively. Quantitative PCR was performed in triplicate according to the custom designed real-time PCR assay from Primerdesign. 18S was used as a housekeeping gene according to the results of the GeNorm kit used in the current study (sense primer sequence: CGGACAGGATTGACAGATTGATA, anti-sense primer: TGCCAGAGTCTCGTTCGTTA).
2.6. Flow cytometry
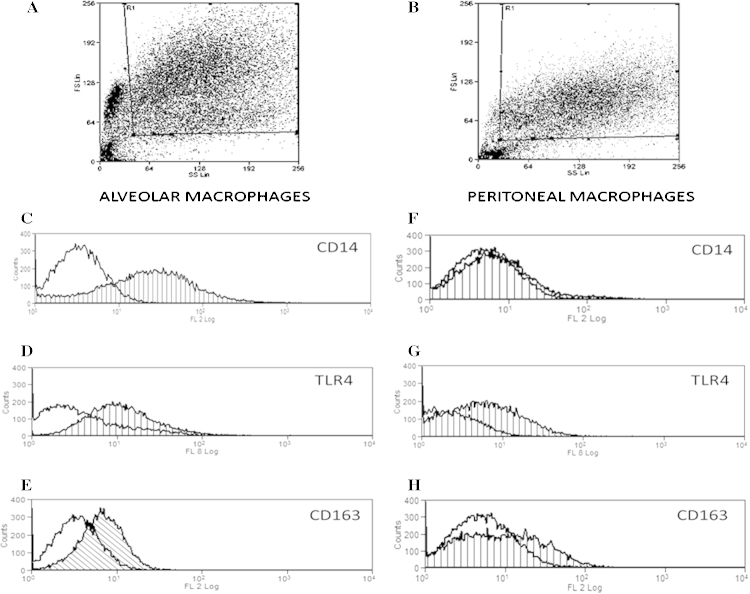
Cells were added to plate wells, as previously described, left overnight and the following morning the medium and non-adherent cells were removed. Remaining adherent cells were gently detached by the use of cell scarpers and 500 μl PBS. One million cells per flow cytometry tube were used for the assay. After 30 min incubation with high-block solution (PBS, 0.1% NaN3, 2% FCS, 0.1%BSA), cells were washed twice with low-block solution (PBS, 0.1% NaN3, 0.2% FCS, 0.1%BSA). Cells were then stained with mouse monoclonal anti-equine CD14 IgG1 Ab (Clone 105, 2 mg/ml, Bettina Wagner Research Lab), goat polyclonal anti-human TLR4 antibody (M-16:sc-12511) and a mouse anti-human macrophage surface Ag monoclonal (AM-3K; Gentaur, product number: KT013) targeting CD163. A goat (Fab2) anti-mouse secondary IgG Ab (RPE Serotec) was used for the CD14 and CD163 Ab staining, while a donkey secondary anti-goat (Alexa-Fluor 633, Life A21082) was used for the TLR4 staining. A negative/isotype control was used for each sample and was stained with only the secondary antibody to control for non-specific binding. Cells were then washed, resuspended in 500 μl of PBS and analysed using CyAn™ ADP Analyser (Beckman Coulter, High Wycombe, UK) and Summit v4.3 software. Data were acquired on 10,000 events and cells gated (Fig. 3A and B) according to size (FS: forward scatter) and granularity (SS: side scatter). Sytox blue dead cell stain (Invitrogen, Catalogue No. S34857) was used in order to measure the viability of cells (97%). Inclusion criteria for the macrophage characterisation were apart from their ability to adhere to the plastic plates, their large size and granularity (forward and side scatter, respectively) observed by flow cytometry (Grünig et al., 1991; Hawkins et al., 1998; Moore et al., 2003; Werners et al., 2004).
Fig. 3.
CD14, CD163 and TLR expression on equine macrophages. Alveolar and peritoneal cells were seeded at 106 cells/ml and left to rest overnight. The next day non-adherent cells were washed away and cells were stained for flow cytometry. Size (FS) and granularity (SS) were used for the cell differentiation and their gating (A and B). Alveolar and peritoneal macrophages were stained for CD14, TLR4 and CD163. Isotype controls are represented by a non-hatched curve and targeting antibody is represented by a hatched curve. Figures are representative of one out of a minimum of 4 experiments. Median fluorescence intensity of the CD14 Ab was 25.88 ± 3.8 for the AMs and 4.96 ± 1.5 for the PMs (C and F). Median fluorescence intensity of the TLR4 was 5.89 ± 1.7 for the AMs and 5.1 ± 2.1 for the PMs (D and G). 11.63 ± 2.6 was the median fluorescence intensity of the AMs and 7.68 for the PMs (E and H). The median fluorescence intensity represents the mean of a minimum of 4 experiments ± SEM.
2.7. Phagocytosis assay
Cells were seeded in duplicate (1 × 106 cells/ml), in Petri dishes (w/2 mm Grid 430196, 60 mm × 15 mm style, Corning Incorporated NY 14831) in complete medium (supplemented only with HS) and left overnight at 37 °C, 5% CO2. The following morning two plates were incubated at 4 °C for at least 30 min. Medium from all plates were refreshed with warm and cold cell culture media as above, respectively, thus, non-adherent cells were removed. Heat-killed Escherichia coli bioparticles (E2861, K-12 strain, 10 mg) were added at a ratio of 10 bacteria for 1 cell in one plate at 37 °C and one at 4 °C. No bacteria were added to the remaining 4 °C and 37 °C plates; these were incorporated to control for autofluorescence. Paired plates were incubated for 1 h at 4 °C and 37 °C, respectively, to allow particle uptake. We measured the number of FITC-labelled Escherichia coli bioparticles in AMs and PMs, which could phagocytose at 37 °C. The 4 °C condition was incorporated to reveal the presence of surface adherent bacteria only, as phagocytosis is inhibited at this temperature. All cells were washed twice with cold PBS and were gently collected with cell scrapers. Cells were then centrifuged at 400 × g for 5 min, resuspended in 500 ml of cold PBS and analysed on a CyAn™ ADP Analyser, as above.
2.8. Statistical analysis
Descriptive statistics were performed using Mini Tab 16 statistical software. After testing for normality, a one-sample Wilcoxon test was employed to identify differences in TNFα production between untreated cells (controls) and cells incubated for 2 h and 6 h after LPs stimulation. Statistical significance was assumed at p < 0.05. Numeric results are presented as mean ± SEM.
3. Results
3.1. Cell recovery and populations
Approximately 2.35 × 108 (±51.6) AMs and 1.33 × 108 (±17.3) PMs were reproducibly isolated from each of 9 systemically healthy horses. Cell viability on the day of cell harvesting was greater than 90% as assessed by Trypan blue (data not shown). Differential cell counts of raw BALF and peritoneal lavage (PL) fluid, as assessed by light microscopy, are summarised in Table 1. Following overnight culture and removal of non-adherent cells, more than 95% of adherent cells were identified morphologically as macrophages after performing a Leishman stain on the culture plates, for both AMs and PMs.
Table 1.
Differential cell count of alveolar and peritoneal lavage.
| Alveolar lavagea | Peritoneal lavagea | |
|---|---|---|
| Macrophages (%) | 73.94 (±3.2) | 54.19 (±6.9) |
| Lymphocytes (%) | 19.43 (±3.0) | 7.94 (±2.2) |
| Neutrophils (%) | 2.73 (±1.1) | 37.81 (±7.8) |
| Mast cells (%) | 3.7 (±4.0) | 0.06 (±0.01) |
| Eosinophils (%) | 0.2 (±0.2) | 0.00 (±0.00) |
Results are the mean of 9 experiments ± SEM.
3.2. Response of AMs and PMs to inflammatory stimulus
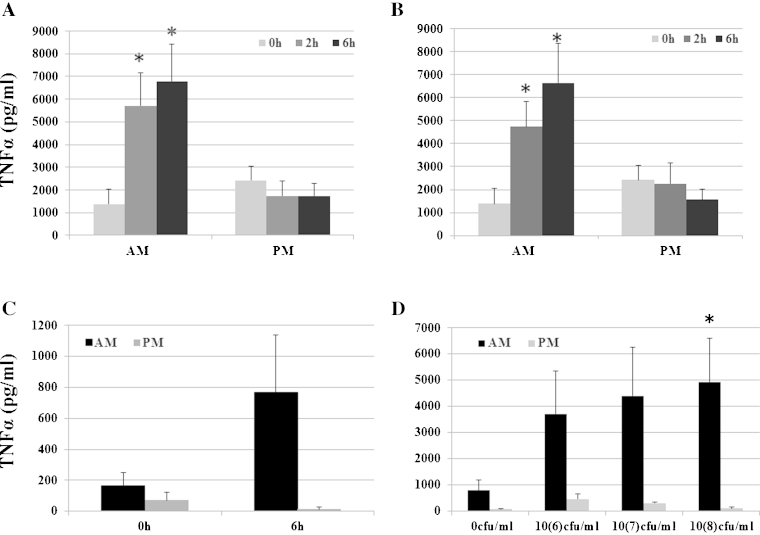
To investigate the relative responsiveness of AMs and PMs to LPS, cells were plated at a concentration of 106 cells/ml and left to rest overnight in HS, as mentioned above. The next day, medium was refreshed; cells were stimulated with LPS. The supernatant concentration of TNFα was measured prior to and 2 h and 6 h following stimulation with LPS. No statistically significant difference was observed between low and high dose of LPS treatment (Fig. 1A and B). LPS-induced TNFα production by AMs was detected by 2 h and was statistically significant higher compared to untreated cells. PMs did not show any LPS-induced TNFα production. Culture supernatant TNFα concentration measured at 24 h post LPS stimulation of AMs was not significantly different from that measured at the 6 h time point, suggesting that a peak in TNFα concentration is reached between 6 h and 24 h (data not shown). At 24 h, there was still no detectable TNFα production by PMs (data not shown). To determine whether the lack of responsiveness of PMs could be attributed to a deficiency in the TLR4 pathway, we stimulated both AMs and PMs with other agonists containing alternative ligands. These included Poly IC (2 μg/ml, a TLR3 ligand) and heat-killed bacteria Salmonella typhimurium (106 cfu/ml, 107 cfu/ml and 108 cfu/ml) that contain a mixture of TLR4 (LPS), TLR2 (peptidoglycan), TLR9 (CpG) and TLR5 ligands (flagelin) (Fig. 1C and D respectively). AMs, but not PMs produced TNFα in response to these stimuli. IL-10 levels remained almost undetectable in both AM and PM at all-time points (data not shown).
Fig. 1.
TNF production of AMs and PMs in response to inflammatory stimuli. Alveolar and peritoneal cells were seeded at 106 cells/ml and left to rest overnight. The next day non-adherent cells were washed away and cell media was refreshed with HS. AMs and PMs were stimulated with 10 and 100 ng/ml of LPS for 0 h, 2 h and 6 h (A and B). AMs and PMs were alsostimulated with 2000 ng/ml of PolyIC for 6 h (B) and with 3 doses of Salmonella thyphimurium (106, 107 and 108 cfu/ml) (C). Results are the mean of a minimum of 4 experiments ± SEM (*p < 0.05 versus control 0 h).
3.3. LPS-induced TNFα and IDO mRNA expression in AMs and PMs
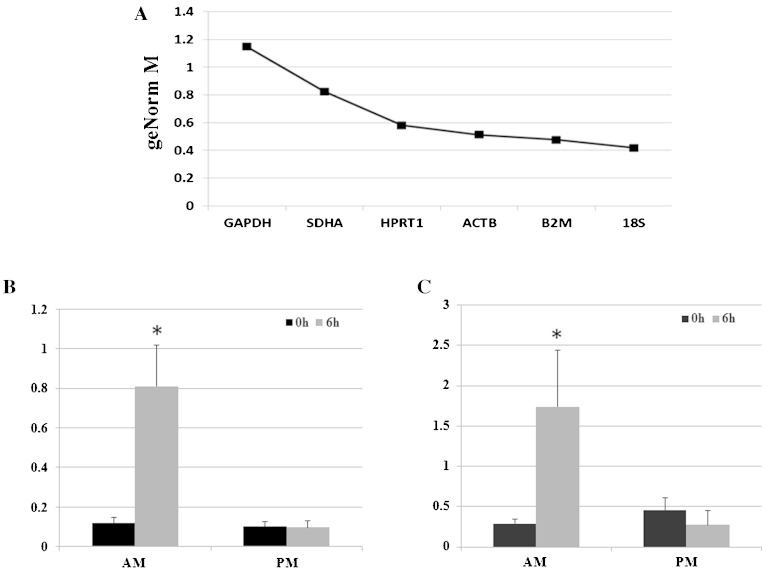
RNA was extracted from AMs and PMs at 0 h and 6 h following LPS (100 ng/ml) stimulation, providing an average yield of 175.82 ng/μl of RNA (±37.9) for AMs and 51.02 ng/μl (±10.1) for PMs. Reference gene selection was based on widely published housekeeping genes used in several horse studies. GAPDH and ACTB were repeatedly used in many experiments performed on BALF derived cells (Laan et al., 2006; Perkins et al., 2008; Riihimaki et al., 2008; Reyner et al., 2009; Beekman et al., 2011; Hughes et al., 2011) and other genes such as HPRT1, SDHA, 18S, B2M, 28S have been investigated in other equine tissue cells (Cappelli et al., 2008; Zhang et al., 2009). In order to select the most appropriate reference gene for the equine macrophage-based studies, we ranked 6 candidate reference genes based on their M value, as assessed using GeNorm (qBaseplus software) (data not shown). An M-value describes the variation of a gene compared to all other candidate genes and the gene with the lowest M-value was considered to be the most stable gene (Vandesompele et al., 2002). We found 18S to be the most stable gene compared to B2M, ACTB, HPRT1 and SDHA (Fig. 2A). Interestingly, we found the commonly used GAPDH to be the least stable gene of the group. In agreement with the results of the TNFα ELISA results, only AMs showed an up-regulation of TNF transcript, which was statistically significant between LPS treated AMs and controls (Fig. 2B). Similarly, IDO expression was also statistically significantly up-regulated in the horse treated AMs but was not expressed in PMs (Fig. 2C).
Fig. 2.
mRNA expression of TNF and IDO production of AMs and PMs in response to LPS. (A) GeNorm (qBaseplus software) was used for the ranking of the six candidate reference genes based on their M value. All reference genes had values less than 1.5 which is the cutoff point under which genes are considered to be stably expressed. 18S was identified as the most stable gene. Quantitative PCR of the TNF (B) and IDO (C) transcripts, respectively, were performed on cDNA samples of both AMs and PMs and mRNA expression was normalised to 18S. Results are the mean of a minimum of 4 experiments ± SEM (*p < 0.05 versus control 0 h).
3.4. CD14, CD163 and TLR4 expression on AMs and PMs
In a further attempt to investigate the lack of LPS-responsiveness of PMs, we evaluated the relative expression of various relevant cell surface markers. These included the LPS receptor TLR4 and its co-receptor CD14. Expression of CD163, a scavenger R and common macrophage marker was also evaluated. Cells were gated, as shown in Fig. 3A and B. AMs highly express CD14, TLR4 and CD163 (Fig. 3C–E). In contrast, PMs although showing an equivalent expression of TLR4 to AMs, expressed very low levels of CD14 and CD163 (Fig. 3F–C).
3.5. Phagocytic properties of AMs and PMs
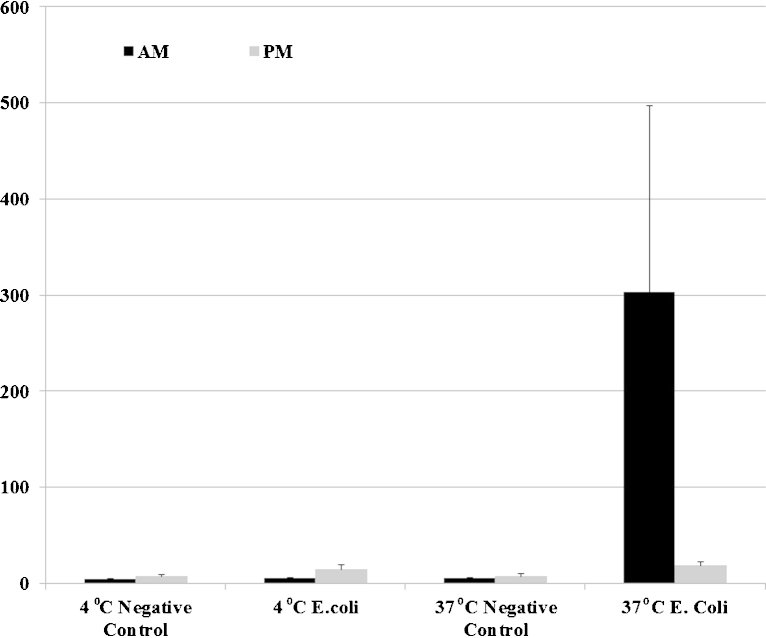
As the morphological assessment of PMs revealed them to be significantly smaller and less ruffled, compared with AMs (data not shown), we further investigated whether this difference in appearance reflected a difference in phagocytic capability. Compared to AMs, PMs phagocytosed a significantly lower number of FITC-labelled E. coli, thus indicating a relative deficiency in their ability internalise despite an apparent ability to bind, bacteria. The average of median fluorescence of the populations (AMs and PMs) at 4 °C and 37 °C is presented in Fig. 4.
Fig. 4.
AMs, but not PMs, can phagocyte bacteria. Alveolar and peritoneal cells were seeded at 106 cells/ml into 2 different plates and left to rest overnight. The next day non-adherent cells were washed away and cell media was refreshed with HS. One plate was left at 4 °C in order to inhibit phagocytosis while the other was left at 37 °C for 30 min. FITC-labelled Escherichia coli at a concentration of 16.5 μg/ml (MOI of 10:1) were added into the wells for 1 h. Cells were then washed twice and analysed by flow cytometry. Control cells at 37 °C and 4 °C, were included in the experiment as well as cells with E. coli are at 37 °C and 4 °C. Mean of the median fluorescence intensity of the 3 experiments ± SEM is shown in the figure AMs are in black bars and PMs in grey bars.
3.6. LPS-induced NO production by AMs and PMs
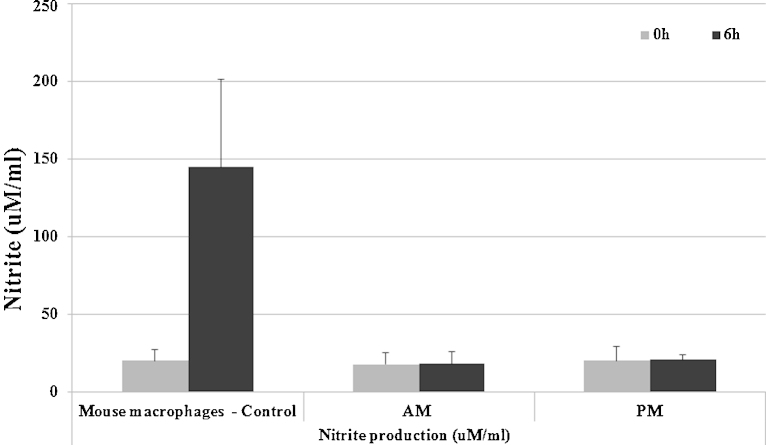
Nitric oxide has been attributed many functions in host defence based largely upon studies in rodents (Ito et al., 2005; Takacs et al., 2012). Hammond et al. reported that equine lung macrophages can induce iNOS, and produce nitric oxide, in response to LPS (Hammond et al., 1999). In order to investigate whether equine-derived macrophages produce NO, we stimulated both AMs and PMs with LPS (100 ng/ml) for 0 h and 6 h and measured the subsequent production of nitrite. As a positive control cell population, we used the mouse macrophage cell line RAW 274.1, known to produce NO in response to LPS. In comparison to the mouse cell line, which produced abundant nitrite, no nitrite was detected in the supernatant of LPS-stimulated equine AMs or PMs (Fig. 5).
Fig. 5.
Production of Nitrite by horse macrophages. Alveolar and peritoneal cells were seeded at 106 cells/ml and left to rest overnight. The next day non-adherent cells were washed away and cell were stimulated by 100 ng/ml of LPS for 0 h and 6 h in presence of HS. Mouse macrophage RAW 274.1 was used as control.
4. Discussion
This study, as well as providing functional and phenotypic data on equine AMs and PMs, has demonstrated significant differences between these cell populations. AMs showed all the characteristics of potent macrophages like CD14, CD163 and TLR4 expression, significant TNFα production after LPS stimulation and phagocytic activity. Work in other species, such as humans and mice has demonstrated a similar phenomenon, highlighting specific characteristic properties of AMs, considered to reflect their unique microenvironment, which support the theory that AMs show a unique phenotype, not observed in other types of macrophages (Gonzalez-Juarrero and Orme, 2001; Guth et al., 2009).
One interesting result was the failure of harvested equine PMs to produce TNFα in response to stimulation with LPS, Poly IC and heat killed bacteria of S. typhimurium. In contrast to our results, Barton et al. did describe TNFα production by equine PMs following LPS stimulation; however, the data presented demonstrated marked variation in the responses and the TNFα detection method differed from our study, relying on the measurement of TNFα activity using a cell line bioassay (Barton et al., 1996). Furthermore, other studies on LPS responsiveness in equine PMs have shown the response to be neither dose nor time dependent (Morris et al., 1990). In order to further investigate this apparent discrepancy in study outcomes, we performed qPCR to measure TNFα mRNA expression in both AMs and PMs, the results of which supported the ELISA data, confirming a lack of TNFα production by equine PMs. In agreement with our findings, several studies in mice PMs have also shown relatively low levels of TNFα release following LPS stimulation (Bradbury and Moreno, 1993; Zheng and Specter, 1996; Sherry et al., 2007; Skuladottir et al., 2007). Indeed, it has been shown that resident murine PMs fail to actively respond to stimuli, necessitating activation with thyoglycolate to induce an inflammatory response (Hetland and Bungum, 1988; Morris et al., 1990; Zheng and Specter, 1996; Nomura et al., 2000). The biological reason for this apparent deficiency in responsiveness of equine PMs is unclear but may reflect a degree of LPS tolerance. Although none of the horses sampled had evidence of gastrointestinal disease, which may have resulted in endotoxin translocation across the intestinal wall, it is possible that low level endotoxin exposure may occur in this species which may also explain the relatively high ratio of neutrophils within the peritoneal cavity of all horses. Furthermore, although PM isolation was not performed in a completely aseptic manner, the likelihood of PM stimulation during the sampling period is low and had this been the case, any LPS tolerance should have been transient and disappeared with overnight cell adherence. However, if a phenomenon of ante mortem LPS tolerance exists in equine PMs, this is evidently not associated with TLR4 down regulation, as reported in murine PMs (Nomura et al., 2000). In contrast to TLR4 expression, the surface expression of CD14 was low in equine PMs, which likely contributed to their lack of LPS responsiveness. IL10, as an anti-inflammatory cytokine, is well documented to inhibit cytokine production, such as IL1, TNFα and IL6 by LPS treated macrophage cell lines and murine peritoneal macrophages (Fiorentino et al., 1991). Similarly, the presence of human IL10 significantly reduced inflammatory mediators, such as IL6, TNF and prostaglandin E2 by equine PMs with a particular effect on TNFα (Hawkins et al., 1998). Therefore, we investigated whether a similar phenomenon was present in equine PMs of the current study, potentially contributing towards their relative LPS non-responsiveness, by measuring IL10 on the cell culture supernatant. However, the IL-10 levels remained almost undetectable in both AMs and PMs at all-time points (data not shown).
Irrespective of the underlying mechanism, it is likely that this state of low LPS responsiveness may be normal for equine PMs despite having a common progenitor cell to AMs (Gorgani et al., 2008; Kapetanovic et al., 2011), consistent with significantly different functional and phenotypic properties. Similar to flow cytometry, morphological differences between AMs and PMs were also observed by light microscopy, supporting differentiation in their function and phenotype. AMs were larger in size, had a greater number of granules within their cytoplasm and developed a greater number of pseudopodia compared to PMs (data not shown).
The greater phagocytic activity and LPS responsiveness of AMs and their surface expression of CD14, CD163 as well as TLR4 likely reflect the microenvironment within which these cells reside and the appropriateness of these inherent biological properties within such an environment. Our detection of TLR4 expression on AMs was in agreement with the results of Suri et al. who reported TLR4 expression in healthy horse lungs (Suri et al., 2006). In the lungs there is a continuous challenge of AMs with airborne agents, which is not the case in the peritoneal cavity. Some similarities between these cell populations were detected, including the cell surface expression of TLR4 and CD163, a scavenger R specific to mononuclear cells. It is likely that the ongoing development and increased availability of novel monoclonal antibodies, such as CD16, should continue to facilitate further comparative studies of different macrophage populations (Noronha et al., 2012). Despite the biological reasons for the difference in the status of AMs and PMs, this data has demonstrated the importance of targeting the tissue of interest when conducting studies on macrophage biology, and not extrapolating from results obtained from cells harvested from disparate anatomical sites.
As we relied on qPCR to support our data, it was considered important to select the most appropriate reference genes for result validation. We discovered the 18S and B2M genes to represent the optimal combination of potential reference genes for studies on LPS-treated AMs, with 18S being the most appropriate single reference gene selection. To date, there have been several attempts to identify reference genes for a variety of equine tissues (Bogaert et al., 2006; Beekman et al., 2011). Our results are concordant with Zhang et al. who found 18S and ACTB to show no significant differences in expression between the following horse tissues: colon, small intestine, heart, spleen, kidney, liver, lung and lymph nodes (Zhang et al., 2009). However in contrast to the findings of Zhang et al., we found B2M to be the second more stable one of our pool of genes (Zhang et al., 2009). Interestingly, in a study of equine airway cells derived from cases of Inflammatory Airway Disease, Beekman et al. recommended using a combination of GADPH, HPRT, SDHA and RPL32, stating GAPDH to be most stable (Beekman et al., 2011). Consequently, optimal housekeeping genes may vary remarkably, depending on cell type, tissues type, species and even disease status, thus highlighting the need for caution when making the appropriate selection to acquire reliable results.
Previous studies have identified phenotypic similarities between human and equine mononuclear cells, including the identification of CD14+CD16+ monocytes in both species, a phenotype not reported in mice (Fingerle et al., 1993; Sunderkotter et al., 2004; Noronha et al., 2012). Similarly, our study has identified functional similarities between the equine and human macrophage, including the up-regulation of IDO and the failure to produce nitrite in response to LPS stimulation. In contrast to an earlier report (Hammond et al., 1999) we found no evidence of LPS-induced nitrite production by both AMs and PMs, a finding which mirrors the response of both porcine and human macrophages (Weinberg et al., 1995; Hawkins et al., 1998; Kapetanovic et al., 2012). We suggest that the difference reflects a major divergence of promoter sequences between the large animals and rodents. Horses, like humans and pigs, have evolved differently to nocturnal animals, such as the mouse, by regulating other anti-bacterial genes like IDO and the vitamin D3 pathway (Thoma-Uszynski et al., 2001; Helenius et al., 2005; Kapetanovic et al., 2012; Korf et al., 2012). In response to LPS, rodent macrophages induce arginine uptake and nitric oxide synthase (NOS) and release nitric oxide (Thoma-Uszynski et al., 2001). This pathway has been widely implicated in pathogen resistance. A previous study revealed that pig and human macrophages also fail to produce nitric oxide in response to LPS, and attributed this difference to a major divergence in the iNOS (NOS2A) promoter sequences between the species (Kapetanovic et al., 2012). We therefore aligned sequences of the iNOS promoter in the three species: human, mouse and horse and found that human and horse promoters are well conserved, whereas there is little alignment of the promoter sequences between mouse/horse and mouse/human (data not shown).
IDO is an enzyme that catalyses tryptophan and its activation leads to a depletion of the amino acid which is essential for bacterial growth. The LPS-induced IDO gene up-regulation we detected mirrors the situation in humans and pigs and supports the findings of previous studies which report IDO up-regulation in horse AMs during intracellular bacterial infection (Heller et al., 2010). Interestingly, IDO gene up-regulation does not occur in murine macrophages despite the mouse being a commonly used model for human inflammatory research. Such fundamental differences in cellular biology have supported the use of other large animals, such as pigs, as models for human inflammatory diseases (Fairbairn et al., 2011; Kapetanovic et al., 2012). Our data demonstrating the similarities between equine and human macrophage biology are potentially supportive of the horse also being regarded as an appropriate model for human inflammatory research involving this cell type. Furthermore, we have demonstrated the horse to be an abundant source of macrophages, which could facilitate such comparative studies by permitting sequential examination of cells harvested from the same individual.
5. Conclusion
In summary, we have identified significant phenotypic and functional differences between equine AMs and PMs, a finding which likely reflects their microenvironment and respective roles in defence against pathogens and tissue homeostasis. We have explored some of the mechanisms underlying the functional difference in these cell populations with regard to LPS responsiveness, which are likely in part due to differences in CD14 expression. This information is crucial to the understanding of the role of macrophages in specific disease states and to the development of therapeutic and/or prophylactic approaches to such, whereby the macrophage is a candidate target cell. Finally, we have demonstrated significant similarities between the equine and human macrophage, supporting the horse as a potentially suitable model for the study of human macrophage biology.
Acknowledgements
The authors would like to acknowledge the University of Edinburg, Pfizer pharmaceutical company and the Biotechnology and Biological Sciences Research Council for funding the current project.
Footnotes
This research project and the contributions of AEK, BM and SP were supported by the Royal (Dick) school of Veterinary studies, Pfizer pharmaceutical company, Moredun Research Institute, the Scottish Agricultural College and the Biotechnology and Biological Sciences Research Council. RK and DAH were supported by the Biotechnology and Biological Sciences Research Council (grant ref: BB/G004013/1).
References
- Barton M.H., Collatos C., Moore J.N. Endotoxin induced expression of tumour necrosis factor, tissue factor and plasminogen activator inhibitor activity by peritoneal macrophages. Equine Vet. J. 1996;28(5):382–389. doi: 10.1111/j.2042-3306.1996.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Beekman L., Tohver T., Dardari R., Leguillette R. Evaluation of suitable reference genes for gene expression studies in bronchoalveolar lavage cells from horses with inflammatory airway disease. BMC Mol. Biol. 2011;12(5) doi: 10.1186/1471-2199-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert L., Van Poucke M., De Baere C., Peelman L., Gasthuys F., Martens A. Selection of a set of reliable reference genes for quantitative real-time PCR in normal equine skin and in equine sarcoids. BMC Biotechnol. 2006;6 doi: 10.1186/1472-6750-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M.G., Moreno C. Effect of lipoarabinomannan and mycobacteria on tumor-necrosis-factor production by different populations of murine macrophages. Clin. Exp. Immunol. 1993;94(1):57–63. doi: 10.1111/j.1365-2249.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelli K., Felicetti M., Capomaccio S., Spinsanti G., Silvestrelli M., Supplizi A.V. Exercise induced stress in horses: selection of the most stable reference genes for quantitative RT-PCR normalization. BMC Mol. Biol. 2008;9 doi: 10.1186/1471-2199-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell J.M. Proceedings of the 4th World Equine Airways Symposium. 2009. Risk factors for inflammatory airway disease in UK National Hunt racehorses. www.ivis.org. [Google Scholar]
- Fairbairn L., Kapetanovic R., Sester D.P., Hume D.A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukocyte Biol. 2011;89(6):855–871. doi: 10.1189/jlb.1110607. [DOI] [PubMed] [Google Scholar]
- Ferrucci F., Zucca E., Croci C., Di Fabio V., Martino P.A., Ferro E. Bacterial pneumonia and pleuropneumonia in sport horses: 17 cases (2001–2003) Equine Vet. Educ. 2008;20(10):526–531. [Google Scholar]
- Fingerle G., Pforte A., Passlick B., Blumenstein M., Strobel M., Zieglerheitbrock H.W.L. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82(10):3170–3176. [PubMed] [Google Scholar]
- Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., Ogarra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147(11):3815–3822. [PubMed] [Google Scholar]
- Gonzalez-Juarrero M., Orme I.M. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect. Immun. 2001;69(2):1127–1133. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgani N.N., Ma Y., Clark H.F. Gene signatures reflect the marked heterogeneity of tissue-resident macrophages. Immunol. Cell Biol. 2008;86(3):246–254. doi: 10.1038/sj.icb.7100131. [DOI] [PubMed] [Google Scholar]
- Gross S.S., Levi R. Tetrahydrobiopterin synthesis – an absolute requirement for cytokine-induced nitric-oxide generation by vascular smooth-muscle. J. Biol. Chem. 1992;267(36):25722–25729. [PubMed] [Google Scholar]
- Grünig G., Hulliger C., Winder C., Hermann M., Jungi T.W., von Fellenberg R. Spontaneous and lipopolysaccharide-induced expression of procoagulant activity by equine lung macrophages in comparison with blood monocytes and blood neutrophils. Vet. Immunol. Immunopathol. 1991;29(3–4):295–312. doi: 10.1016/0165-2427(91)90021-4. [DOI] [PubMed] [Google Scholar]
- Guth A.M., Janssen W.J., Bosio C.M., Crouch E.C., Henson P.M., Dow S.W. Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296(6):L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme J. Release of tumor necrosis factor-alpha by human peritoneal-macrophages in vivo and in vitro. Am. J. Obstet. Gynecol. 1989;161(6):1718–1725. doi: 10.1016/0002-9378(89)90957-5. [DOI] [PubMed] [Google Scholar]
- Hammond R.A., Hannon R., Frean S.P., Armstrong S.J., Flower R.J., Bryant C.E. Endotoxin induction of nitric oxide synthase and cyclooxygenase-2 in equine alveolar macrophages. Am. J. Vet. Res. 1999;60(4):426–431. [PubMed] [Google Scholar]
- Hawkins D.L., MacKay R.J., MacKay S.L.D., Moldawer L.L. Human interleukin 10 suppresses production of inflammatory mediators by LPS-stimulated equine peritoneal macrophages. Vet. Immunol. Immunopathol. 1998;66(1):1–10. doi: 10.1016/s0165-2427(98)00181-0. [DOI] [PubMed] [Google Scholar]
- Helenius I., Lumme A., Haahtela T. Asthma, airway inflammation and treatment in elite athletes. Sports Med. 2005;35(7):565–574. doi: 10.2165/00007256-200535070-00002. [DOI] [PubMed] [Google Scholar]
- Heller M.C., Drew C.P., Jackson K.A., Griffey S., Watson J.L. A potential role for indoleamine 2,3-dioxygenase (IDO) in Rhodococcus equi infection. Vet. Immunol. Immunopathol. 2010;138(3):174–182. doi: 10.1016/j.vetimm.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Hetland G., Bungum L. Human peritoneal-macrophages – production invitro of the active terminal complement components C5 to C9 and a functional alternative pathway of complement. APMIS. 1988;96(1):89–92. [PubMed] [Google Scholar]
- Hoffman A.M. Bronchoalveolar lavage technique and cytological diagnosis of small airway inflammatory disease. Equine Vet. Educ. 1999;11(6):330–336. [Google Scholar]
- Hughes K.J., Nicolson L., Da Costa N., Franklin S.H., Allen K.J., Dunham S.P. Evaluation of cytokine mRNA expression in bronchoalveolar lavage cells from horses with inflammatory airway disease. Vet. Immunol. Immunopathol. 2011;140(1–2):82–89. doi: 10.1016/j.vetimm.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Hume D.A. Macrophages as APC and the dendritic cell myth. J. Immunol. 2008;181(9):5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- Hume D.A., Ross I.L., Himes S.R., Sasmono R.T., Wells C.A., Ravasi T. The mononuclear phagocyte system revisited. J. Leukocyte Biol. 2002;72(4):621–627. [PubMed] [Google Scholar]
- Ito H., Koide N., Morikawa A., Hassan F., Islam S., Tumurkhuu G., Mori I., Yoshida T., Kakumu S., Moriwaki H., Yokochi T. Augmentation of lipopolysaccharide-induced nitric oxide production by alpha-galactosylceramide in mouse peritoneal cells. J. Endotoxin Res. 2005;11(4):213–219. doi: 10.1179/096805105X46628. [DOI] [PubMed] [Google Scholar]
- Kapetanovic R., Fairbairn L., Beraldi D., Sester D.P., Archibald A.L., Tuggle C.K., Hume D.A. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J. Immunol. 2012;188(7):3382–3394. doi: 10.4049/jimmunol.1102649. [DOI] [PubMed] [Google Scholar]
- Kapetanovic R., Parlato M., Fitting C., Quesniaux V., Cavaillon J.-M., Adib-Conquy M. Mechanisms of TNF induction by heat-killed Staphylococcus aureus differ upon the origin of mononuclear phagocytes. Am. J. Physiol. 2011;300(4):C850–C859. doi: 10.1152/ajpcell.00187.2010. [DOI] [PubMed] [Google Scholar]
- Katakami Y., Nakao Y., Koizumi T. Regulation of tumor necrosis factor production by mouse peritoneal-macrophages – the role of cellular cyclic-AMP. Immunology. 1988;64(4):719–724. [PMC free article] [PubMed] [Google Scholar]
- Korf H., Wenes M., Stijlemans B., Takiishi T., Robert S., Miani M., Eizirik D.L., Gysemans C., Mathieu C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217(12):1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Laan T., Bull S., Pirie S., Fink-Gremmels J. Evaluation of cytokine production by equine alveolar macrophages exposed to lipopolysaccharide, Aspergillus fumigatus, and a suspension of hay dust. Am. J. Vet. Res. 2005;66(9):1584–1589. doi: 10.2460/ajvr.2005.66.1584. [DOI] [PubMed] [Google Scholar]
- Laan T.T.J.M., Bull S., Pirie R., Fink-Gremmels J. The role of alveolar macrophages in the pathogenesis of recurrent airway obstruction in horses. J. Vet. Intern. Med. 2006;20(1):167–174. doi: 10.1892/0891-6640(2006)20[167:troami]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Prause O., Sjostrand M., Laan M., Lotvall J., Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 2003;170(9):4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- Moore B.D., Balasuriya U.B.R., Watson J.L., Bosio C.M., MacKay R.J., Maclachlan N.J. Virulent and avirulent strains of equine arteritis virus induce different quantities of TNF-α and other proinflammatory cytokines in alveolar and blood-derived equine macrophages. Virology. 2003;314(2):662–670. doi: 10.1016/s0042-6822(03)00506-3. [DOI] [PubMed] [Google Scholar]
- Morris D.D., Crowe N., Moore J.N., Moldawer L.L. Endotoxin-induced production of interleukin-6 by equine peritoneal-macrophages in vitro. Am. J. Vet. Res. 1992;53(8):1298–1301. [PubMed] [Google Scholar]
- Morris D.D., Moore J.N., Fischer K., Tarleton R.L. Endotoxin-induced tumor-necrosis-factor activity production by equine peritoneal macrophages. Circ. Shock. 1990;30(3):229–236. [PubMed] [Google Scholar]
- Nomura F., Akashi S., Sakao Y., Sato S., Kawai T., Matsumoto M., Nakanishi K., Kimoto M., Miyake K., Takeda K., Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 2000;164(7):3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- Noronha L.E., Harman R.M., Wagner B., Antczak D.F. Generation and characterization of monoclonal antibodies to equine CD16. Vet. Immunol. Immunopathol. 2012;146(2):135–142. doi: 10.1016/j.vetimm.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G.A., Viel L., Wagner B., Hoffman A., Erb H.N., Ainsworth D.M. Histamine bronchoprovocation does not affect bronchoalveolar lavage fluid cytology, gene expression and protein concentrations of IL-4, IL-8 and IFN-gamma. Vet. Immunol. Immunopathol. 2008;126(3–4):230–235. doi: 10.1016/j.vetimm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Reyner C.L., Wagner B., Young J.C., Ainsworth D.M. Effects of in vitro exposure to hay dust on expression of interleukin-23,-17,-8, and-1 beta and chemokine (C-X-C motif) ligand 2 by pulmonary mononuclear cells from horses susceptible to recurrent airway obstruction. Am. J. Vet. Res. 2009;70(10):1277–1283. doi: 10.2460/ajvr.70.10.1277. [DOI] [PubMed] [Google Scholar]
- Riihimaki M., Raine A., Pourazar J., Sandstrom T., Art T., Lekeux P., Couetil L., Pringle J. Epithelial expression of mRNA and protein for IL-6, IL-10 and TNF-alpha in endobronchial biopsies in horses with recurrent airway obstruction. BMC Vet. Res. 2008;4 doi: 10.1186/1746-6148-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N.E., Hoffman A. Inflammatory airway disease: defining the syndrome, Conclusions of the Havemeyer Workshop. Equine Vet. Educ. 2003;5:81–84. [Google Scholar]
- Sherry C.L., O’Connor J.C., Kramer J.M., Freund G.G. Augmented lipopolysaccharide-induced TNF-alpha production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 MAPK. J. Immunol. 2007;178(2):663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- Skuladottir I.H., Petursdottir D.H., Hardardottir I. The effects of omega-3 polyunsaturated fatty acids on TNF-alpha and IL-10 secretion by murine peritoneal cells in vitro. Lipids. 2007;42(8):699–706. doi: 10.1007/s11745-007-3081-1. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C., Nikolic T., Dillon M.J., van Rooijen N., Stehling M., Drevets D.A., Leenen P.J.M. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Suri S.S., Janardhan K.S., Parbhakar O., Caldwell S., Appleyard G., Singh B. Expression of Toll-like receptor 4 and 2 in horse lungs. Vet. Res. 2006;37(4):541–551. doi: 10.1051/vetres:2006017. [DOI] [PubMed] [Google Scholar]
- Takacs A.C., Swierzy I.J., Lueder C.G.K. Interferon-gamma restricts toxoplasma gondii development in murine skeletal muscle cells via nitric oxide production and immunity-related GTPases. PLoS ONE. 2012;7(9.) doi: 10.1371/journal.pone.0045440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma-Uszynski S., Stenger S., Takeuchi O., Ochoa M.T., Engele M., Sieling P.A., Barnes P.F., Rollinghoff M., Bolcskei P.L., Wagner M., Akira S., Norgard M.V., Belisle J.T., Godowski P.J., Bloom B.R., Modlin R.L. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291(5508):1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. 0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J.B., Misukonis M.A., Shami P.J., Mason S.N., Sauls D.L., Dittman W.A., Wood E.R., Smith G.K., McDonald B., Bachus K.E., Haney A.F., Granger D.L. Human mononuclear phagocyte inducible nitric-oxide synthase (INOS) – analysis of INOS messenger-RNA, INOS protein, biopterin, and nitric-oxide production by blood monocytes and peritoneal-macrophages. Blood. 1995;86(3):1184–1195. [PubMed] [Google Scholar]
- Werners A.H., Bull S., Fink-Gremmels J., Bryant C.E. Generation and characterisation of an equine macrophage cell line (e-CAS cells) derived from equine bone marrow cells. Vet. Immunol. Immunopathol. 2004;97(1–2):65–76. doi: 10.1016/j.vetimm.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Wlaschitz S., Scherzer S. Equine bacterial pleuropneumonia. Pferdeheilkunde. 2000;16(4):367. [Google Scholar]
- Wood J.L.N., Newton J.R., Chanter N., Mumford J.A. Inflammatory airway disease, nasal discharge and respiratory infections in young British racehorses. Equine Vet. J. 2005;37(3):236–242. doi: 10.2746/0425164054530579. [DOI] [PubMed] [Google Scholar]
- Zhang X.K., Morrison D.C. Lipopolysaccharide-induced selective priming effects on tumor-necrosis-factor-alpha and nitric-oxide production in mouse peritoneal macrophages. J. Exp. Med. 1993;177(2):511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.W., Davis E.G., Bai J. Determination of internal control for gene expression studies in equine tissues and cell culture using quantitative RT-PCR. Vet. Immunol. Immunopathol. 2009;130(1–2):114–119. doi: 10.1016/j.vetimm.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Zheng Z.M., Specter S. Dynamic production of tumour necrosis factor-alpha (TNF-alpha) messenger RNA, intracellular and extracellular TNF-alpha by murine macrophages and possible association with protein tyrosine phosphorylation of STAT1 alpha and ERK2 as an early signal. Immunology. 1996;87(4):544–550. doi: 10.1046/j.1365-2567.1996.513591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]