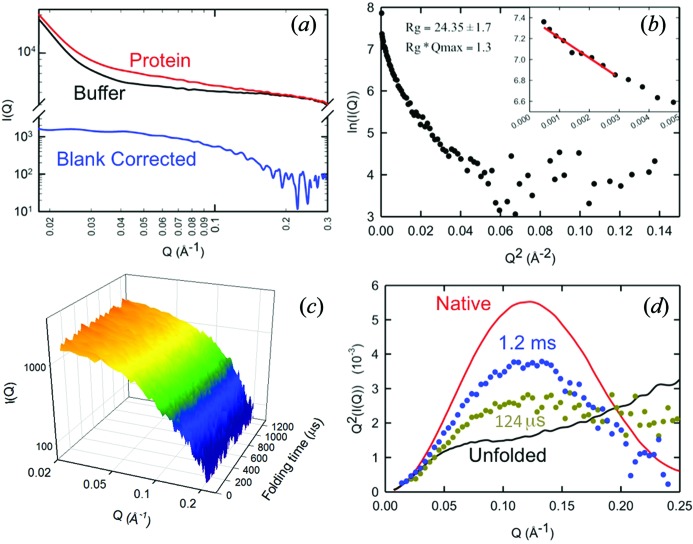
Figure 4.
Representative continuous-flow micro-SAXS data collected on horse heart cytochrome c. Refolding from the random-coil-like state (4.5 M GdnHCl) was initiated by tenfold dilution of GdnHCl with buffer using the continuous-flow mixer. (a) Raw data for protein (red) and buffer (black) at a representative time point (100 µs) along the channel. The blank subtracted data are shown in blue. (b) Representative Guinier fit of the data (100–148 µs points averaged). The solid red line is a weighted least-squares Guinier fit. (c) The blank-corrected scattering curves for 3.5 mg ml−1 cytochrome c over the 0.1–1.2 ms time range after initiation of folding. Final conditions are 0.45 M GdnHCl, 0.2 M imidazole and pH 7.0. Each scattering curve is the average of approximately ten frames of 200 ms exposure. (d) Kratky plots at representative time points compared with measurements taken under equilibrium conditions for folded (red) and unfolded (black) cytochrome c. Data from 100 to 148 µs were averaged for a representative plot of the beginning of the channel (gold), and 2.33–2.40 ms were averaged for a representative plot of the end of the channel (blue). Each plot is normalized to I 0.