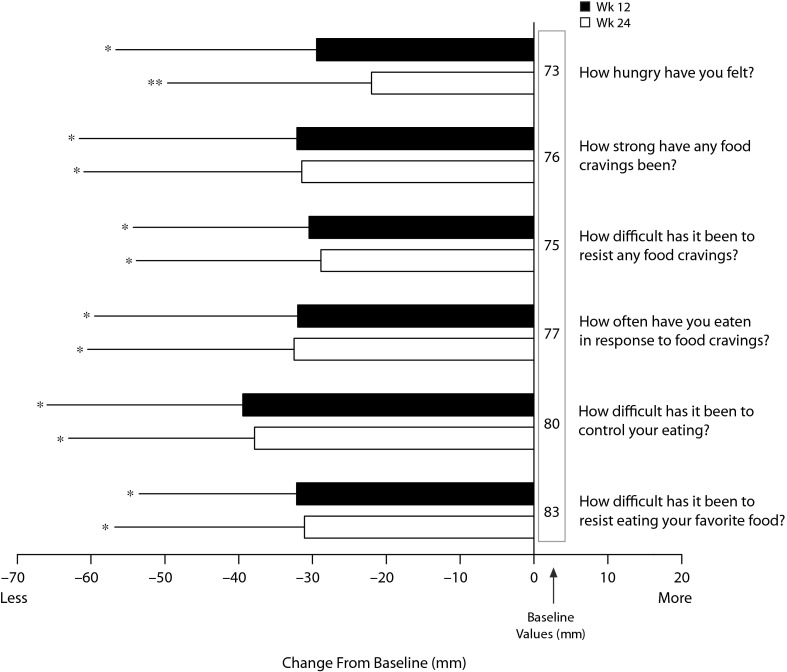
Figure 2.
Improvement in Selected Eating and Food Craving Items on the Control of Eating Questionnaire After 12 and 24 Weeks of Treatment With NB32a,b
aModified intent-to-treat–last observation carried forward; data represent mean ± SD. Responses reflect experiences during the previous 7 days.
bP values are based on a t test that assesses if mean change from baseline is significantly different from zero.
*P < .001.
**P < .01.
Abbreviation: NB32 = naltrexone sustained-release 32 mg/bupropion sustained-release 360 mg.