Abstract
The scaling relationship between leaf dry mass and leaf surface area has important implications for understanding the ability of plants to harvest sunlight and grow. Whether and how the scaling relationships vary across environmental gradients are poorly understood. We analyzed the scaling relationships between leaf mass and leaf area of 121 vascular plant species along an altitudinal gradient in a subtropical monsoon forest. The slopes increased significantly with altitude, it varied from less than 1 at low altitude to more than 1 at high altitude. This means that plants growing at high altitude allocate proportionately more biomass to support tissues in larger leaves and less in smaller leaves, whereas the reverse is true at low altitude. This pattern can be explained by different leaf strategies in response to environmental pressure and constrains.
Introduction
Leaf dry mass (M) and leaf surface area (A) are two important leaf traits of the vast majority of vascular plants [1]. The relative changes in these two parameters can be described as a ‘power law’, mathematically taking the form: M = βAα, where β is the normalization constant and α is the scaling exponent [2], [3]. This formula reveals that SLA, the light-capturing surface built by the plant per unit investment of dry mass, is size-dependent. Since SLA = A/M and M = βAα, it follows that SLA = (1/β)A1-α. The value of α>1 indicates that larger leaves have lower SLA than smaller ones, whereas α<1 means the opposite. If M scales isometrically to A (i.e. α = 1), then changes in leaf size have no impact on SLA. Quantifying the scaling relationships between leaf size and SLA will improve our understanding of how leaves maintain a positive carbon balance and influence whole plant fitness.
Ecologists have reported empirical evidences with respect to SLA and leaf size. Several studies have shown that as leaves increase in mass, increases in surface area often fail to keep pace with the increases in mass (i.e. α>1) [2], [4], which has been called “diminishing returns”. Alternatively, it has been noted that SLA increased with leaf size, which yields “increasing returns” (i.e. α<1) [4]–[6]. Both phenomena probably occur due to different biomass distribution between productive and support tissues in large compared to small leaves [7]–[9]. Additionally, it was also found that leaf mass scales isometrically to leaf area (i.e. α = 1) [4], [10], this is size-independent and results in a “break even” relationship.
Within the leaves, there are at least two components: an expanded lamina (i.e. productive tissues) and a beam-like petiole (i.e. support tissues) [11]. Leaf biomass partitioning is an important driver of whole-plant net carbon gain. Plant growth rate scales positively with the mass fraction in leaf lamina and is negatively associated with the fraction of support tissues [7]. Some researches indicate that leaf size modifies the distribution of leaf biomass between productive and support tissues [2], [4], which further leads to the underlying allometric scaling relationships between M and A (i.e. α>1 or α<1).
Environmental factors may influence the relationship between M and A. Plant modularity has allowed plant to optimize resource distribution among different structures [12]. Optimal allocation theory predicts that plants should invest more biomass to the compartment that acquires the most limiting resource to adapt to environmental changes [13]. For example, in some extreme environments such as strong wind, compared to smaller leaves, larger leaves may increase the proportion of biomass allocation to lamina support tissues to provide sufficient mechanical stability [14]. Plants adapted to such unfavorable environments will exhibit M–A slopes>1 (i.e. diminishing returns). Furthermore, M–A slopes would vary with the environmental gradients as leaves adopt different biomass allocation strategies in response to environment changes. Prior studies have examined the M-A scaling relationships among and within species [2], [4], [10], and whether and how the scaling relationships vary across environmental gradients are still poorly understood.
Along altitudinal gradients, the environment changes rapidly over short distances. Plants subject to lower temperature, higher irradiance and strong wind at higher altitude, while light limits at lower altitude. Herein we investigated the effects of altitude on the scaling relationship between M and A in a subtropical monsoon forest. The specific questions we asked were (i) What are the relationships between M and A at different altitudes? (ii) Whether the slope of the log M–log A relationship change with increasing elevation, and if so, how?
Materials and Methods
Ethics Statement
This research was approved by the Administration Bureau of Mt. Tianmu Nature Reserve in China.
Study Site
This study was conducted in Mt. Tianmu Nature Reserve, the north subtropical area of eastern China (30° 19.61′–30° 28.90′ N, 119° 25.67′–119° 26.41′ E). The altitude varies from 350 m to 1506 m. Mean annual precipitation ranges from 1390 mm at lower elevations to 1870 mm at higher elevations, and mean annual air temperature decreases from 14.8°C at the foot of the mountain to 8.8°C at the top. The reserve has obvious vertical vegetation zones, including evergreen broad-leaved forest (350–850 m), evergreen and deciduous broad-leaved mixed forest (850–1100 m), deciduous broad-leaved forest (1100–1380 m), and dwarf forest (1380–1506 m).
Sampling
Six collection sites were chosen at ca. 200 m elevation intervals along the elevation in May 2012 (Table 1). A total of 121 vascular plant species were sampled. At each site, the most abundant plant species were selected. For each species, leaves were gathered from at least three different adult individuals which are similar-sized and not shaded by neighboring plants, and then bulked into a single sample. Petioles were included in leaf area and mass measurements. The petioles in simple-leaved species were assumed to be analogous to the sum of petioles, rachises, and petiolules in compound-leaved species [7]. Leaf area was measured with a leaf area meter (CI-203, Laser leaf area meter CID, Inc. USA). Dry mass was determined after oven-drying at 70°C for at least 72 h.
Table 1. Scaling exponents (α) and intercepts (β) of M-A relationship at six altitudes as estimated by RMA regression.
| Altitude (m) | Latitude (N°) | Longitude (E°) | α | 95% CI of α | β | 95% CI of β | R2 | Sample size |
| 414 | 119°26.41′ | 30°19.61′ | 0.859 c | 0.780, 0.946 | −2.103 | −2.249, −1.957 | 0.823 | 78 |
| 620 | 119°26.24′ | 30°19.90′ | 0.963bc | 0.853, 1.088 | −2.248 | −2.463, −2.033 | 0.642 | 97 |
| 850 | 119°26.00′ | 30°20.24′ | 1.000b | 0.912, 1.097 | −2.374 | −2.541, −2.206 | 0.704 | 138 |
| 1086 | 119°26.03′ | 30°24.49′ | 1.113ab | 0.974, 1.273 | −2.473 | −2.541, −2.206 | 0.640 | 81 |
| 1286 | 119°25.67′ | 30°20.64′ | 1.299 a | 1.160, 1.455 | −2.868 | −3.114, −2.623 | 0.773 | 72 |
| 1462 | 119°25.51′ | 30°28.90′ | 1.258 a | 1.161, 1.364 | −2.821 | −2.993, −2.649 | 0.839 | 99 |
All regressions were significant (P<0.05). Boldfaced slopes are statistically significant different from 1 at P<0.05 level. The Post-hoc multiple comparison of slopes were shown among altitudes, where the slopes sharing the same superscript letters are not significantly different from each other at P<0.05 level.
Statistical Analysis
The data for A and M were log-transformed. Since functional rather than predictive relationships were sought [15], reduced major axis (RMA, also called standardized major axis) regression was used to determine the scaling exponent and constant of log–log linear functions. Differences in the regression slopes among altitudes were tested by multiple post hoc comparisons. The significance level for testing slope heterogeneity and difference from slope = 1 was P<0.05. All of the analyses were conducted using SMATR Version 2.0 [16].
Results
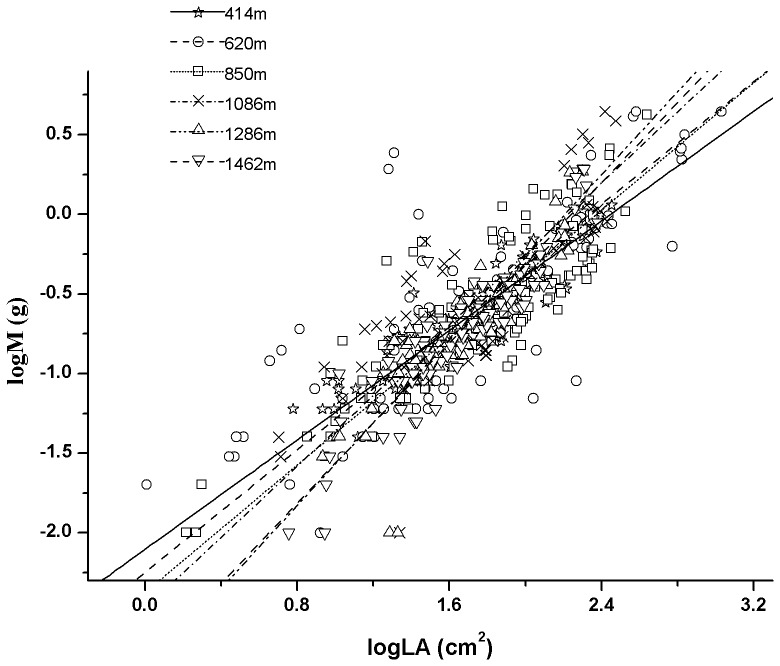
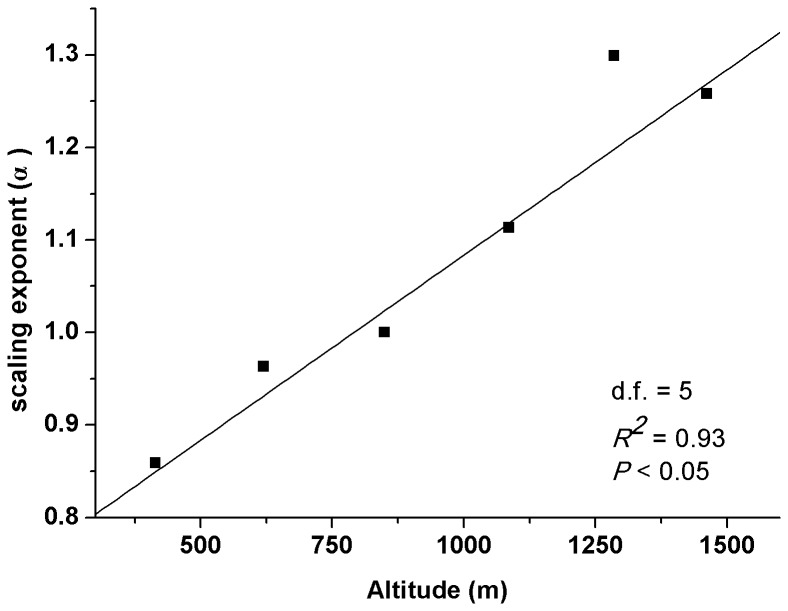
There was significant positive relationship between leaf mass and leaf area at each altitude (Table 1, Fig. 1). The slopes showed a great degree of variability among altitudes, ranging from 0.859 to 1.299. The slopes were significantly<1 at low altitude (414 m), whereas significantly>1 at high altitudes (1286 m and 1462 m). In middle altitudes, the slopes were not significantly different from 1 (620 m, 850 m and 1086 m). The post hoc multiple comparisons of slopes among altitudes showed that slopes at high altitudes were significantly higher than those at low altitude (Table 1). Furthermore, there was a highly significant positive relationship between the estimated slopes and altitude (Fig. 2).
Figure 1. Leaf mass-area relationship at six sites as estimated by RMA regression.
Figure 2. Scaling exponent (α) of the leaf mass-area relationship increases with altitude.
Discussion
The M-A scaling relationship has important implications for understanding the ability of plants to acclimate to environmental conditions [17]. In the present study, we found a wide range of variation in M to A scaling exponents among altitudes (from 0.859 to 1.299) (Fig. 1, 2). This is consistent with results from prior theoretical and empirical studies in which no constant value was used to describe the M-A relationships across all leaves [2], [12], [17]. Environmental controls on specific leaf area induces the variant leaf allometry [9]. Leaves are subject to strong selective gradients in aridity, solar radiation, and nutrient availability that affect their size and shape [12], [18]. Accordingly, the scaling values are expected to vary across environments as they balance the need for efficient conductance, net carbon acquisition, and protection against desiccation.
The scaling of M vs. A affects leaf economy in size-dependent ways, which has an important implication for leaf size optimization [11]. In this study, M scales ‘faster’ than A, and larger leaves show lower SLA than smaller ones at high altitudes (i.e. α>1) (Fig. 1, 2). Plant performances are limited by low temperature, high irradiance and strong wind at high altitudes. Leaf support structure provides laminas with both mechanical support and a pathway for water and nutrient transport. Low temperature would limit the transportation efficiency and thus, leaves may require a high investment in the transporting structure [11]. High irradiance and strong wind would increase the proportion of biomass allocation to leaf support structure [19]–[21]. In particular, large leaves require rigid support and mechanic resistance because they suffer large drag forces and static loads [20], [22]. Therefore, large leaves tend to have a larger fractional biomass investment in support structure relative to small ones. Small leaves, on the other hand, produce smaller wind-induced drag forces and have lower support needs, and thus a higher fraction of productive tissue. This diminishing return in the scaling of leaf size with leaf support investment implies that small leaves with greater SLA are more likely to be favoured at higher altitudes.
In our study, SLA increases disproportionately with increasing A at low altitude (i.e. α<1) (Fig. 1, 2). Light is a key limiting resource for plant growth and survival at low altitude [23]. Plants grown under low light intensities tend to have low photosynthetic capacities per unit leaf area [24], [25], thus, plants would evolve to maximize the biomass allocation to laminas and minimize the lamina support investment to capture more light. Leaf size has important consequences for the scale and precision with which plants forage for light. Large leaves at low light intensities may intercept a large amount of light due to their more extensive foliar display, whereas small leaves are better able to exploit fine-grained environmental heterogeneity by positioning their leaves in light-rich micro-patches [26]. It has also been reported that leaf shape, leaf angle and petiole length alter leaf light-interception efficiency [27]. Small leaves regular their leaf arrangement to fills the gaps via modifications in petiole length and thus to take advantage of the penumbra effect. Hence, more investment in petioles may radically change light-interception capacity of small leaves. However, efficient light harvesting via supporting structural modifications may become increasingly expensive with increasing leaf size [7]. Large leaves may manifest an adaptive modification towards avoiding enhanced costs for leaf support, and may consequently construct cheaper and more extensive light-intercepting foliar display than small leaves. Thus, plants may optimise a pay-off of having large leaves and efficient light intercepting surface and high SLA with low investment of photosynthesising tissues per unit area at low altitude. Environmental conditions at middle altitudes are in between low and high altitude, which thus lead to the isometric relationship between M and A (i.e. α = 1) (Fig. 1, 2). Our results imply that the differences in size-dependent SLA may be an adaptive response to limits imposed on plant growth and survival by environmental conditions.
The scaling relationship reflects the results of an evolutionary trade-off among many ancestral metabolic, morphological, and anatomical traits shared by all vascular plants [2]. Our research here exclusively focused on the altitude gradient, and therefore in-depth understanding of the developmental mechanisms underlying allometric strategies across other environmental gradient (e.g., aridity) requires further exploration.
Acknowledgments
We thank Dr. Jianjun Tang and Mingshui Zhao for assistance in the field investigation, Dr. Kun Jiang for critical comments on the manuscript, and Prof. De’an Jiang for providing the leaf area meter.
Funding Statement
The study was financially supported by Zhejiang Provincial Natural Science Foundation of China (LZ13C030002), Natural Science Fund of China (31330010) and Ministry of Science and Technology of China (2011AA100503). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Calvo-Alvarado JC, McDowell NG, Waring RH (2008) Allometric relationships predicting foliar biomass and leaf area: sapwood area ratio from tree height in five Costa Rican rain forest species. Tree physiology 28: 1601–1608. [DOI] [PubMed] [Google Scholar]
- 2. Niklas KJ, Cobb ED, Niinemets Ü, Reich PB, Sellin A, et al. (2007) “Diminishing returns” in the scaling of functional leaf traits across and within species groups. Proceedings of the National Academy of Sciences 104: 8891–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niklas KJ, Christianson ML (2011) Differences in the scaling of area and mass of Ginkgo biloba (Ginkgoaceae) leaves and their relevance to the study of specific leaf area. American Journal of Botany 98: 1381–1386. [DOI] [PubMed] [Google Scholar]
- 4. Milla R, Reich PB (2007) The scaling of leaf area and mass: the cost of light interception increases with leaf size. Proceedings of the Royal Society B: Biological Sciences 274: 2109–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niinemets Ü, Kull K (1994) Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. Forest Ecology and Management 70: 1–10. [Google Scholar]
- 6. Ackerly DD, Reich PB (1999) Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. American Journal of Botany 86: 1272–1281. [PubMed] [Google Scholar]
- 7. Niinemets Ü, Portsmuth A, Tobias M (2006) Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytologist 171: 91–104. [DOI] [PubMed] [Google Scholar]
- 8. Niinemets Ü, Portsmuth A, Tena D, Tobias M, Matesanz S, et al. (2007) Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Annals of Botany 100: 283–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Milla R, Reich P, Niinemets Ü, Castro-Díez P (2008) Environmental and developmental controls on specific leaf area are little modified by leaf allometry. Functional Ecology 22: 565–576. [Google Scholar]
- 10. Shipley B (1995) Structured interspecific determinants of specific leaf area in 34 species of herbaceous angiosperms. Functional Ecology 9: 312–319. [Google Scholar]
- 11. Li G, Yang D, Sun S (2008) Allometric relationships between lamina area, lamina mass and petiole mass of 93 temperate woody species vary with leaf habit, leaf form and altitude. Functional Ecology 22: 557–564. [Google Scholar]
- 12. Price CA, Enquist BJ (2007) Scaling mass and morphology in leaves: an extension of the WBE model. Ecology 88: 1132–1141. [DOI] [PubMed] [Google Scholar]
- 13. Zhang WP, Jia X, Bai YY, Wang GX (2011) The difference between above- and below-ground self-thinning lines in forest communities. Ecol Res 26: 819–825. [Google Scholar]
- 14. Takenaka A, Takahashi K, Kohyama T (2001) Optimal leaf display and biomass partitioning for efficient light capture in an understorey palm, Licuala arbuscula . Functional Ecology 15: 660–668. [Google Scholar]
- 15.Niklas KJ (1994) Plant allometry: the scaling of form and process. Chicago: University of Chicago Press.
- 16.Falster D, Warton D, Wright I (2006) User’s guide to SMATR: standardised major axis tests and routines version 2.0, copyright 2006. Available: http://www.bio.mq.edu.au/ecology/SMATR/. Accessed 2013 May 3.
- 17. Niklas KJ, Cobb ED, Spatz H-C (2009) Predicting the allometry of leaf surface area and dry mass. American Journal of Botany 96: 531–536. [DOI] [PubMed] [Google Scholar]
- 18. Tomlinson KW, Poorter L, Sterck FJ, Borghetti F, Ward D, et al. (2013) Leaf adaptations of evergreen and deciduous trees of semi-arid and humid savannas on three continents. Journal of Ecology 101: 430–440. [Google Scholar]
- 19.Niklas KJ (1992) Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago press.
- 20. Niklas KJ (1999) A mechanical perspective on foliage leaf form and function. New Phytologist 143: 19–31. [Google Scholar]
- 21. Niinemets Ü, Kull O (1999) Biomass investment in leaf lamina versus lamina support in relation to growth irradiance and leaf size in temperate deciduous trees. Tree physiology 19: 349–358. [DOI] [PubMed] [Google Scholar]
- 22.Givnish TJ (1986) On the Economy of Plant Form and Function: Proceedings of the Sixth Maria Moors Cabot Symposium, “Evolutionary Constraints on Primary Product Ivity, Adaptive Patterns of Energy Capture in Plants”, Harvard Forest, August 1983: Cambridge University Press.
- 23. Zhang WP, Jia X, Morris EC, Bai YY, Wang GX (2012) Stem, branch and leaf biomass-density relationships in forest communities. Ecol Res 27: 819–825. [Google Scholar]
- 24. Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. [DOI] [PubMed] [Google Scholar]
- 25. Niinemets Ü (2007) Photosynthesis and resource distribution through plant canopies. Plant, Cell & Environment 30: 1052–1071. [DOI] [PubMed] [Google Scholar]
- 26. Poorter L, Rozendaal DM (2008) Leaf size and leaf display of thirty-eight tropical tree species. Oecologia 158: 35–46. [DOI] [PubMed] [Google Scholar]
- 27. Niinemets Ü, Al Afas N, Cescatti A, Pellis A, Ceulemans R (2004) Petiole length and biomass investment in support modify light interception efficiency in dense poplar plantations. Tree physiology 24: 141–154. [DOI] [PubMed] [Google Scholar]