Abstract
Identifying the genetic basis of complex diseases, such as rheumatoid arthritis, remains a challenge that requires experimental models to reduce the genetic and environmental variability. Numerous loci for arthritis have been identified in induced animal models; however, few spontaneous models have been genetically studied. Therefore, we generated a four-way advanced intercross line (AIL) from four inbred strains, including BXD2/TyJ which spontaneously develops autoimmune arthritis. A genome-wide scan for spontaneous arthritis was performed in a cohort of 366 mice of the fourth generation (G4) of this cross. Five loci contributing to clinical phenotypes were identified in chromosomes 3, 7, 13, 18, and X. Three of the loci found in this study, confirm previously identified loci; whereas two of them are novel loci. Interesting candidate genes for the loci are highlighted. This study provides a genetic overview of spontaneous arthritis in mice and aids to solve the genetic etiology of rheumatoid arthritis and to gain a better understanding of the disease.
Introduction
Rheumatoid arthritis (RA) is a systemic chronic autoimmune inflammatory disease that primarily affects joints. Genetic factors have a strong impact in RA susceptibility and development [1]. The heritability of RA is estimated to be around 60% [2]. The most important genetic risk is the human leukocyte antigen (HLA) locus. Its influence is estimated to be one third of the overall genetic susceptibility to RA [2], but it confers susceptibility only to autoantibodies to citrullinated protein antigens (ACPA)-positive patients [3].
The knowledge of genes and pathways involved in disease is of great importance to understand the pathogenic mechanisms of disease, and consequently to improve therapy, diagnosis and disease prevention. Linkage and association studies in human are commonly used to identify candidate susceptibility loci in Mendelian disorder. However, the heterogeneity of the human genome, the minor single gene contribution to the pathogenesis, and the multiplex gene-gene and gene-environment interactions render challenging to identify genes with low effect in complex disease. Therefore, animal models are invaluable tools to decipher genetic factors affecting quantitative traits, since they allow us to control the genetic background and to define the environmental conditions.
BXD2/TyJ is a recombinant inbred strain generated by inbreeding for more than 20 generations a F2 intercross of the strains C57BL/6J and DBA/2J [4]. BXD2/TyJ mice spontaneously develop chronic erosive arthritis and generalized autoimmune disease. Mice start to develop arthritis after 4 months, and between 9 and 12 months 66% of females and 42% of males are affected. BXD2/TyJ mice produce high titers of rheumatoid factor (RF) and antibodies against DNA, with predominance of IgG1 and IgG2b isotypes. Those mice also demonstrate glomerulonephritis, proteinuria, and splenomegaly [5]. BXD2/TyJ strain develops features of autoimmune diseases due to a complex combination of interacting genes inherited from the original parental strains, C57BL/6J and DBA/2J, which develop neither arthritis nor lupus. These characteristics make the BXD2/TyJ strain an exceptional model to study genetics of autoimmune diseases such as erosive arthritis.
There are different strategies to identify loci controlling quantitative traits (so called quantitative trait loci, QTLs) in murine animal model. The traditional mapping in a cross between two inbred strains (F2 intercross and N2 backcross) is restricted by the low resolution and the limited genetic variability. An advanced intercross line (AIL) is produced by random and sequential intercrossing of two or more inbred strains for many generations by avoiding brother–sister mating, so that the progeny carries a recombinant genome from the parental strains [6]. An AIL offers a higher genomic resolution than conventional F2 and N2 crosses due to the accumulation of a greater density of recombination events, and therefore, is a powerful approach to identify and further refine QTLs.
Here, the genetic determinants of the BXD2/TyJ strain were studied in a four-way autoimmune-prone AIL generated from four inbred strains. A whole-genome scan was performed in a cohort of mice from the four generation of the AIL, and several genomic regions controlling different phenotypes were identified.
Materials and Methods
Mice
An outbred four-way autoimmune-prone advanced intercross line (AIL) was generated by our group from the parental mouse strains BXD2/TyJ, MRL/MpJ, NZM2410/J and CAST/EiJ, all acquired from The Jackson Laboratory (Bar Harbor, ME). The four inbred strains were intercrossed following an equal strain and sex distribution. At each time, 50 breeding pairs were used to generate the next generation. A total of 366 mice from the fourth generation (G4) were enrolled in this study and evaluated for 6 months. Mice were assessed once a week to evaluate clinical disease according to a scoring system based on the number of inflamed joints [7]. Each paw was scored individually, each inflamed toe and knuckle was given a score of 1, and an inflamed wrist or ankle was given a score of 5; maximum score of 15 per limb and of 60 per mouse. Mice were bred and housed under climate-controlled conditions with 12-h light/darkness cycles at the animal facility at the University of Rostock. The procedures were approved by the governmental administration of the State of Mecklenburg-Vorpommern.
Genotyping
Pure genomic DNA was isolated with the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. Illumina Mouse MD Linkage Panel was used to genotype 1,449 SNPs, of which 1,199 were polymorphic between the parental strains. The average distance between informative markers was 1.77 Mbp.
QTL mapping
Single locus linear model
The association analysis was performed by the R version of HAPPY [8] on Debian Linux [9]. In brief, the founder haplotype structure for each mouse is inferred by the HAPPY algorithm taking into account the adjacent markers. The association seeks for differences between the genetic effects of the parental haplotypes. QTLs then are detected by a regression model applied to the inferred haplotypes. This association provides ANOVA significance levels, presented as the negative base-10 logarithmic P value (-log P). To determine the empirical threshold for statistical significance, 1,000 permutations were performed [8]. An empirical significance threshold was established at P<0.001 for each phenotype. Data from all chromosomes were analyzed simultaneously with an additive model.
Some of the mice in this study were simultaneously assessed for autoimmune skin blistering disease (epidermolysis bullosa acquisita, EBA) development for an independent study, and therefore immunized with type VII collagen (ColVII) [10]. To exclude a bias in the analysis, ColVII immunization and sex were included as covariates. Confidential intervals (CI) of QTLs were estimated manually to comprise the region around a peak up to a drop of the P value by P<0.05.
Efficient mixed-model
To correct for family structure and genetic relatedness among mice and reduce the inflation of false positives, a variance component model approach was used. The Efficient Mixed-Model Association eXpedited (EMMAX) beta version [11] was used for testing association between individual markers and the phenotype. This software works in a computationally efficient manner by avoiding repetitive variance component estimation methods, since it considers that each locus explains only a small fraction of the complex trait. Therefore, a kinship matrix is generated by using Balding-Nicholas procedure in emma-kin function, and then the matrix is used as additional random variable while performing the SNP associations.
Genotype and phenotype of each mouse are provided in Table S1.
Results
The G4 mice of the four-way autoimmune-prone advanced intercross line developed spontaneous arthritis
The outbred four-way autoimmune-prone advanced intercross line (AIL) was originated in our group from the parental mouse strains BXD2/TyJ, MRL/MpJ, NZM2410/J and CAST/EiJ. In the fourth generation (G4), 366 mice were monitored to evaluate clinical arthritis for 6 months. G4 mice developed spontaneous arthritis in 85.8% of males and 57.3% of females. Onset of disease, which reflects the speed of disease progression, and severity of disease, which is measured as maximum score, were similar in both genders (Table 1). A lower percentage of these mice also developed autoimmune pancreatitis [12] and lupus nephritis (data not published).
Table 1. Phenotypic characteristics of spontaneous arthritis in G4 mice from the four-way autoimmune-prone AIL.
| Incidence | Onseta | Maximum scoreb | |
| Females | 113/197 | 22.9 ± 0.4 | 4.5 ± 0.4 |
| Males | 145/169 | 21.9 ± 0.4 | 6.9 ± 0.4 |
| Total | 258/366 | 22.3 ± 0.3 | 5.6 ± 0.3 |
a. Onset measured in weeks. Mean ± standard error of the mean; only disease mice were included in the calculation.
b. Mean ± standard error of the mean; all mice were included in the calculation.
Five loci associated with arthritis phenotypes in the G4 of the four-way autoimmune-prone advanced intercross line were identified
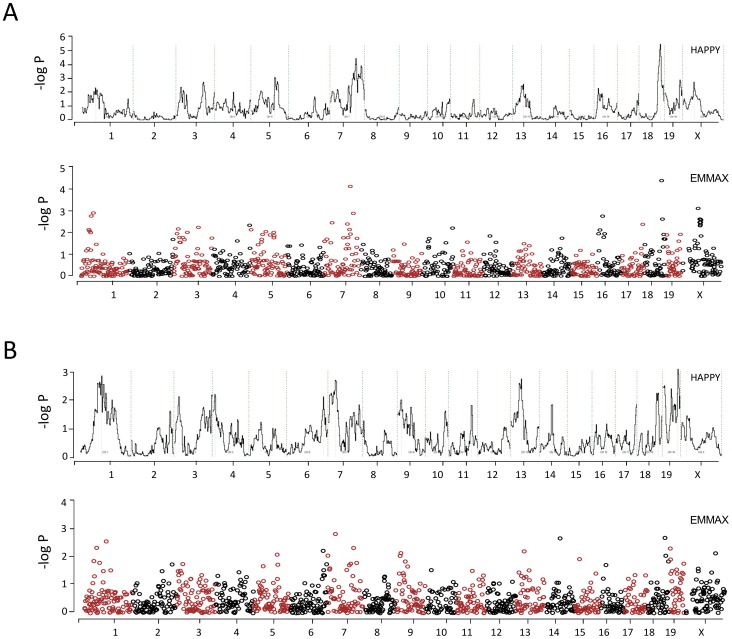
The genetic control of susceptibility and maximum score was analyzed by an association study performed with the software package HAPPY. Results are represented in Figure 1. Statistical significance was based on ANOVA test (-log P), and correspond nicely with the empirical significance levels as assessed by 1,000 permutations for every SNP. A cutoff of 0.001 in the empirical P value, equivalent to one false positive per 1,000 associated SNPs, was defined and those SNPs above this threshold were considered significant. Empirical P values <0.01 were considered suggestive.
Figure 1. Whole-genome association map for spontaneous arthritis traits.
Phenotypes are (A) susceptibility to disease, and (B) disease severity, measured as maximum score. Each graph represents the strength of association between phenotype and marker, using HAPPY (linear plot) or EMMAX (Manhattan plot), for the whole set of 366 G4 mice tested. Gender and ColVII immunization were used as covariates. The x-axis indicates the SNP's chromosomal position, and the y-axis shows the -log P value of association.
HAPPY is the method commonly used for association studies in heterogeneous stocks. It associates haplotypes from the parental strains with the phenotypes and is considered more powerful than single-point methods to identify loci in complex murine crosses since provides information from the parental strains [8]. However, this method does not take into account the genetic relatedness of the mice. It is known that in complex mice crosses as AILs and heterogeneous stocks, family structure and genetic relatedness are important source of false positive findings [13]. Mixed models have the power to effectively exclude relatedness from the analysis. Therefore, we used EMMAX [11], a modification of EMMA which have been successfully used to identify loci in previous human and animal studies, to confirm the loci identified by HAPPY and to exclude false positives. This method associates individual SNPs, instead of haplotypes as HAPPY, with the phenotype, and has the property of include kinship as variable. Statistical significance was also assessed by permutation assay. Loci which reached significance level by both methods were considered significant. Loci with high association by one of the methods and suggestive association in the other were considered suggestive.
Five loci associated with susceptibility or maximum score were found in the screen (Table 2). Two loci were significantly associated with susceptibility to disease on chromosomes 7 and 18, and two loci showed suggestive evidence of association with the same phenotype on chromosome 3 and X (Figure 1 A). Maximum score showed suggestive evidence of association to one locus on chromosomes 13 (Figure 1 B). The loci on chromosome 7, 18 and X were located in previously mapped arthritis QTLs (Table 2); while the locus on chromosome 3 spanned a region without previously mapped autoimmune QTLs. The chromosome 13 QTL did not overlap with known arthritic loci, but with loci controlling disease in models of other human autoimmune diseases.
Table 2. Identified loci for susceptibility and maximum score.
| Chr | Phenotype | HAPPY peak | Position (Mbpa) | Peak -log P | Confidential interval | EMMAX peak | Peak -log P | Published arthritic loci | Ref. |
| 18 | Susceptibility | rs13483436 | 64,67 | 5.4* | rs3688789–rs13483466 | rs6161154 | 4.4* | Pgia11, Cia18 | [17] |
| 7 | Susceptibility | rs3707067 | 105,86 | 4.4* | rs6213614–rs3716088 | rs3713052 | 4.1* | Cia7, Pgia3, Pgia21 | [24] [18] [17] |
| X | Susceptibility | rs3157124 | 68,69 | 2.7# | rs13483765–rs3725966 | rs13483825 | 3.1* | Pgia24 | [17] |
| 3 | Susceptibility | rs3659988 | 16,35 | 2.3* | rs6248752–rs6235984 | rs6235984 | 2.2# | ||
| 13 | Max. score | rs13481764 | 36,64 | 2.6* | rs6275055–rs13481783 | rs3725187 | 2.2# |
a. Position according to the NCBI Build 37.
Empirical P<0.001; # empirical P<0.01.
Chr, chromosome; Mbp, megabase pair; max. score, maximum score.
Discussion
RA is considered rather a syndrome than a discrete disease with a single etiologic source [14]. In fact, RA has already been divided according to the presence or absence of ACPA [15]. This division is also reflected in the genetic heterogeneity and clinical phenotype: HLA-DRB1 and PTPN22 loci are exclusively associated with ACPA-positive patients who, in addition show more severe and destructive disease than ACPA-negative patients [16]. It is noteworthy that BXD2/TyJ mice present RF in peripheral blood. Together with the fact that arthritis develops spontaneously, this strain may be considered a specific model for autoantibody-positive RA.
In this study, the most extensive genetic analysis of BXD2/TyJ spontaneous arthritis was generated. Five arthritis loci were identified, of which two had not been implicated in any previous genetic studies in arthritis.
The family structure of complex crosses such AILs complicates the association analysis, potentially leading to many false positive findings. Therefore, we used a software to account for relatedness. Although no QTL was fine-mapped in sufficient detail to identify the causal genetic variant, potential candidate genes within the loci are highlighted below.
One of the most strongly associated loci maps towards the telomeric end of chromosome 18 and controls susceptibility to disease. This locus spans the previously identified Pgia11 and Cia18 loci, which are associated with susceptibility to arthritis and autoantibody production, respectively [17], [18]. This locus also overlaps with loci associated with the murine models of multiple sclerosis (Eae25) [19], systemic lupus erythematosus (Lbw6) [20], type I diabetes (Idd21.1) and autoimmune ankylosing spondylitis (Pgis1) [21]. The importance of chromosome 18 in susceptibility to autoimmunity in different species had already been reported [22]. In fact, the QTL identified in this study contains genes whose human orthologous had previously been associated with RA in a genome-wide association study (GWAS) [23] such as PTPN2 (protein tyrosine phosphatase, non-receptor type 2, lymphoid), TCF4 (transcription factor 4), ZBTB7C (zinc finger and BTB domain containing 7C), IMPA2 (inositol(myo)-1(or 4)-monophosphatase 2), ATP9B (ATPase class II type 9B or macrophage MHC receptor 1), DYM (dymeclin), CTIF (CBP80/20-dependent translation initiation factor), and CCDC11 (coiled-coil domain containing 11). More compelling candidates within the locus are Nfatc1 gene encoding calcineurin-dependent nuclear factor 1 of activated T cells; Smad2, Smad7 and Smad4 genes encoding proteins from the SMAD (similar to mothers against decapentaplegic) family which mediates TGF-β signaling; Dcc gene encoding a netrin 1 receptor, a member of the immunoglobulin superfamily of cell adhesion molecules; and Malt1 gene encoding a caspase-like protein involved in B-cell and T-cell receptor signaling pathways.
On chromosome 7, a locus strongly associated with susceptibility was found. This locus overlaps with Cia7 [24], Pgia3 [18], and Pgia21 [17], known loci which contribute to induced arthritis; and with loci controlling other experimental autoimmune disease such as Eae4 [25], Nba3 [26], Eae26 [27], and Il4ppq [28]. This QTL has also conservation of synteny with a human region containing genes associated with arthritis such as PRKCB1 (protein kinase C, beta 1) and PDE2A (phosphodiesterase 2A, cGMP-stimulated) [29]. Other candidate genes of particular interest within the locus are the genes encoding the interleukins IL18BP, IL21 and IL27.
On chromosome X, one locus had suggestive association with susceptibility to disease. This association explains at least part of the strong sex effect on arthritis susceptibility. Accordingly, the phenotype maximum score for which no sex effect was found, did not show association with this locus. The region matches with the previously mapped genetic locus Pgia24 controlling antibody response [17]. Plausible biological candidates are Ikbkg gene encoding the NF-κB essential modulator, NEMO, which regulates the activation of NF-κB; and Irak1 gene encoding the interleukin-1 receptor-associated kinase 1.
A locus on chromosome 3 was suggestively associated with susceptibility to disease. This novel QTL was not previously associated with any autoimmune phenotype; however, it harbors potential candidate genes such as Pde7a which encodes a protein from the cyclic nucleotide phosphodiesterase (PDE) family with a relevant role in immune cell activation [30].
The locus that showed suggestive association with maximum score on chromosome 13 overlaps with two loci, Bxs6 [31] and Idd14 [32], controlling different clinical phenotypes in model of autoimmune nephritis and type I diabetes, respectively. The human corresponding region contained the gene CDKAL1 which was associated with RA in a GWAS (rs1459047, [33]). Interesting genes within the locus are Bmp6 coding a protein from the bone morphogenetic protein (BMP) family with known function in cartilage and bone formation and possible role in B-cell differentiation to plasmablasts [34]; and RIPK1 coding a serine-threonine kinase involved in necroptosis and inflammation [35].
The unique integration of genomes from four strains may explain the observation of two novel QTLs for arthritis, i.e. not found previously in other crosses, among the five identified (Table 2). On the other hand, our results show that it is likely that there are common pathways involved in different autoimmune diseases, since some of the QTLs identified in this study overlap with loci controlling autoimmune phenotypes in other mouse models.
The fact that human loci associated with RA in GWAS had corresponding loci in this cross indicates that it is likely that there are important common genes and pathways involved in arthritis in both humans and animals, particularly in the BXD2/TyJ strain. This is robust evidence to confirm that the approach followed in this study is appropriate and powerful to study genetic factor determining human RA in a hypothesis-free manner.
In summary, the present study demonstrates the utility of the generated four-way autoimmune-prone AIL to identify loci affecting arthritis. Two QTLs significantly associated with clinical arthritis and three QTLs with a suggestive association were successfully identified. This study confirms QTLs previously found in other arthritis models and identifies new risk loci for experimental arthritis. Further, fine mapping within each QTL combined with functional studies will be required to identify the causal genes and the pathways leading to disease.
Supporting Information
Genotype-phenotype of the G4 mice of the four-way autoimmune-prone advanced intercross.
(XLSX)
Acknowledgments
We would like to thank Ilona Klamfuss for help with animal work.
Funding Statement
This research was supported by grants from DFG (DFG IB 24/6-1,DFG GRK 1727/1), Excellence Cluster Inflammation at Interfaces (DFG EXC 306/1-2), and BMBF (IMPAM 01EC1008F). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Seldin MF, Amos CI, Ward R, Gregersen PK (1999) The genetics revolution and the assault on rheumatoid arthritis. Arthritis Rheum 42: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 2. MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, et al. (2000) Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43: 30–37. [DOI] [PubMed] [Google Scholar]
- 3. Deighton CM, Walker DJ, Griffiths ID, Roberts DF (1989) The contribution of HLA to rheumatoid arthritis. Clin Genet 36: 178–182. [DOI] [PubMed] [Google Scholar]
- 4. Taylor BA, Wnek C, Kotlus BS, Roemer N, MacTaggart T, et al. (1999) Genotyping new BXD recombinant inbred mouse strains and comparison of BXD and consensus maps. Mamm Genome 10: 335–348. [DOI] [PubMed] [Google Scholar]
- 5. Mountz JD, Yang P, Wu Q, Zhou J, Tousson A, et al. (2005) Genetic segregation of spontaneous erosive arthritis and generalized autoimmune disease in the BXD2 recombinant inbred strain of mice. Scand J Immunol 61: 128–138. [DOI] [PubMed] [Google Scholar]
- 6. Darvasi A, Soller M (1995) Advanced intercross lines, an experimental population for fine genetic mapping. Genetics 141: 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmdahl R, Carlsen S, Mikulowska A, Vestberg M, Brunsberg U, et al.. (1998) Genetic analysis of murine models for rheumatoid arthritis. In: Adolph K, editor. Human Genome Methods. New York. pp. 215–238. [Google Scholar]
- 8. Mott R, Talbot CJ, Turri MG, Collins AC, Flint J (2000) A method for fine mapping quantitative trait loci in outbred animal stocks. Proc Natl Acad Sci U S A 97: 12649–12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moller S, Krabbenhoft HN, Tille A, Paleino D, Williams A, et al. (2010) Community-driven computational biology with Debian Linux. BMC Bioinformatics 11 Suppl 12S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludwig RJ, Muller S, Marques A, Recke A, Schmidt E, et al. (2011) Identification of quantitative trait loci in experimental epidermolysis bullosa acquisita. J Invest Dermatol 132: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 11. Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, et al. (2010) Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asghari F, Fitzner B, Holzhuter SA, Nizze H, de Castro Marques A, et al. (2011) Identification of quantitative trait loci for murine autoimmune pancreatitis. J Med Genet 48: 557–562. [DOI] [PubMed] [Google Scholar]
- 13. Valdar W, Holmes CC, Mott R, Flint J (2009) Mapping in structured populations by resample model averaging. Genetics 182: 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Firestein GS (2005) Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol 11: S39–44. [DOI] [PubMed] [Google Scholar]
- 15. Weyand CM, Klimiuk PA, Goronzy JJ (1998) Heterogeneity of rheumatoid arthritis: from phenotypes to genotypes. Springer Semin Immunopathol 20: 5–22. [DOI] [PubMed] [Google Scholar]
- 16. Imboden JB (2009) The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol 4: 417–434. [DOI] [PubMed] [Google Scholar]
- 17. Adarichev VA, Valdez JC, Bardos T, Finnegan A, Mikecz K, et al. (2003) Combined autoimmune models of arthritis reveal shared and independent qualitative (binary) and quantitative trait loci. J Immunol 170: 2283–2292. [DOI] [PubMed] [Google Scholar]
- 18. Otto JM, Cs-Szabo G, Gallagher J, Velins S, Mikecz K, et al. (1999) Identification of multiple loci linked to inflammation and autoantibody production by a genome scan of a murine model of rheumatoid arthritis. Arthritis Rheum 42: 2524–2531. [DOI] [PubMed] [Google Scholar]
- 19. Blankenhorn EP, Butterfield RJ, Rigby R, Cort L, Giambrone D, et al. (2000) Genetic analysis of the influence of pertussis toxin on experimental allergic encephalomyelitis susceptibility: an environmental agent can override genetic checkpoints. J Immunol 164: 3420–3425. [DOI] [PubMed] [Google Scholar]
- 20. Kono DH, Burlingame RW, Owens DG, Kuramochi A, Balderas RS, et al. (1994) Lupus susceptibility loci in New Zealand mice. Proc Natl Acad Sci U S A 91: 10168–10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vegvari A, Szabo Z, Szanto S, Nesterovitch AB, Mikecz K, et al. (2005) Two major interacting chromosome loci control disease susceptibility in murine model of spondyloarthropathy. J Immunol 175: 2475–2483. [DOI] [PubMed] [Google Scholar]
- 22. Merriman TR, Cordell HJ, Eaves IA, Danoy PA, Coraddu F, et al. (2001) Suggestive evidence for association of human chromosome 18q12-q21 and its orthologue on rat and mouse chromosome 18 with several autoimmune diseases. Diabetes 50: 184–194. [DOI] [PubMed] [Google Scholar]
- 23. WTCCC (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang HT, Jirholt J, Svensson L, Sundvall M, Jansson L, et al. (1999) Identification of genes controlling collagen-induced arthritis in mice: striking homology with susceptibility loci previously identified in the rat. J Immunol 163: 2916–2921. [PubMed] [Google Scholar]
- 25. Baker D, Rosenwasser OA, O'Neill JK, Turk JL (1995) Genetic analysis of experimental allergic encephalomyelitis in mice. J Immunol 155: 4046–4051. [PubMed] [Google Scholar]
- 26. Xie S, Chang SH, Sedrak P, Kaliyaperumal A, Datta SK, et al. (2002) Dominant NZB contributions to lupus in the (SWR x NZB)F1 model. Genes Immun 3 Suppl 1S13–20. [DOI] [PubMed] [Google Scholar]
- 27. Jirholt J, Lindqvist AK, Karlsson J, Andersson A, Holmdahl R (2002) Identification of susceptibility genes for experimental autoimmune encephalomyelitis that overcome the effect of protective alleles at the eae2 locus. Int Immunol 14: 79–85. [DOI] [PubMed] [Google Scholar]
- 28. Shiroiwa W, Tsukamoto K, Ohtsuji M, Lin Q, Ida A, et al. (2007) IL-4Ralpha polymorphism in regulation of IL-4 synthesis by T cells: implication in susceptibility to a subset of murine lupus. Int Immunol 19: 175–183. [DOI] [PubMed] [Google Scholar]
- 29. Okada Y, Terao C, Ikari K, Kochi Y, Ohmura K, et al. (2012) Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat Genet 44: 511–516. [DOI] [PubMed] [Google Scholar]
- 30. Castro A, Jerez MJ, Gil C, Martinez A (2005) Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: advances in the development of specific phosphodiesterase inhibitors. Med Res Rev 25: 229–244. [DOI] [PubMed] [Google Scholar]
- 31. Rankin J, Boyle JJ, Rose SJ, Gabriel L, Lewis M, et al. (2007) The Bxs6 locus of BXSB mice is sufficient for high-level expression of gp70 and the production of gp70 immune complexes. J Immunol 178: 4395–4401. [DOI] [PubMed] [Google Scholar]
- 32. McAleer MA, Reifsnyder P, Palmer SM, Prochazka M, Love JM, et al. (1995) Crosses of NOD mice with the related NON strain. A polygenic model for IDDM. Diabetes 44: 1186–1195. [DOI] [PubMed] [Google Scholar]
- 33. Plenge RM, Cotsapas C, Davies L, Price AL, de Bakker PI, et al. (2007) Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet 39: 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huse K, Bakkebo M, Oksvold MP, Forfang L, Hilden VI, et al. (2011) Bone morphogenetic proteins inhibit CD40L/IL-21-induced Ig production in human B cells: differential effects of BMP-6 and BMP-7. Eur J Immunol 41: 3135–3145. [DOI] [PubMed] [Google Scholar]
- 35. Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38: 209–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genotype-phenotype of the G4 mice of the four-way autoimmune-prone advanced intercross.
(XLSX)