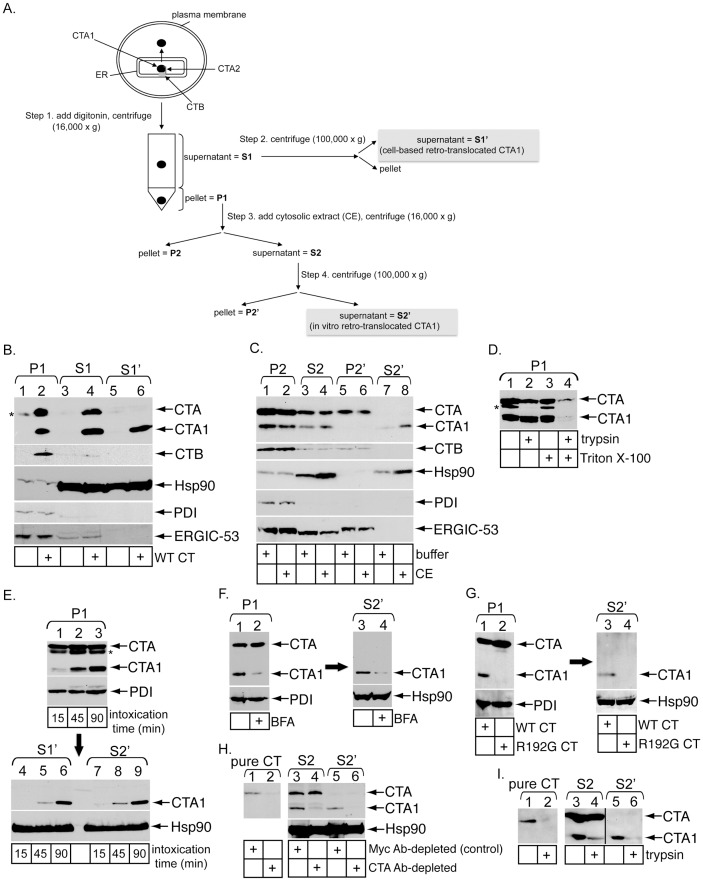
Figure 1. Establishment of an in vitro cholera toxin retro-translocation assay.
(A) Flow diagram of the in vitro retro-translocation assay. Steps 1–4 indicate generation of unique fractions, including P1, S1, S1′, P2, S2, P2′, and S2′. (B) 293T cells were intoxicated with or without 10 nM CT for 45 minutes, permeabilized with digitonin (0.01%), and processed as described in A to generate P1, S1, and S1′. Fractions were analyzed by immunoblotting with the indicated antibodies. * denotes an unidentified protein in the P1 that cross-reacts with the CTA antibody. (C) CT-intoxicated P1 was resuspended in buffer or a cytosolic extract (CE). After incubation for 30 minutes at room temperature, samples were processed to generate P2, S2, P2′, and S2′. Fractions were analyzed by immunoblotting with the indicated antibodies. (D) CT-intoxicated P1 was resuspended in buffer with or without 1% Triton X-100 and with or without 0.3 mg/ml trypsin. Proteolysis proceeded at 4°C for one hour before reactions were stopped with TLCK and samples analyzed by immunoblotting with CTA antibody. * denotes an unidentified protein in the P1 that cross-reacts with the CTA antibody. (E) Cells were incubated at 4°C for 30 minutes in the presence of 20 nM CT to allow binding of toxin to the plasma membrane, washed to remove excess toxin, and then incubated at 37°C to synchronize toxin uptake. Intoxicated cells were harvested after 15, 45 or 90 minutes and subjected to the in vitro retro-translocation assay. P1, S1′ and S2′ fractions were analyzed by immunoblotting with the indicated antibodies. (F) Cells were treated with vehicle or BFA prior to and during intoxication. Intoxicated cells were subjected to the in vitro retro-translocation assay to generate P1 and the corresponding S2′. P1 and S2′ were analyzed by immunoblotting with the indicated antibodies. (G) Cells intoxicated with WT or R192G CT were subjected to the in vitro retro-translocation assay to generate P1 and the corresponding S2′. These fractions were analyzed by immunoblotting with the indicated antibodies. (H) Purified CTA, S2 and S2′ were incubated with the control Myc antibody or an antibody against CTA. Immune-complexes were precipitated using protein A agarose beads and the resulting supernatants analyzed by immunoblotting with the indicated antibodies. (I) Purified CTA, S2 and S2′ were incubated with buffer or 0.3 mg/ml trypsin at 4°C for one hour before reactions were stopped with TLCK and analyzed by immunoblotting with CTA antibody.