Abstract
Accurate and reliable measurement of intraocular pressure (IOP) is crucial in the study of glaucoma using the mouse model. The purpose of this study was to determine the relationship between TonoLab-measured IOP and central corneal thickness (CCT) in mouse strains with single gene mutations of matricellular proteins. Wild-type (WT) and transgenic mouse strains with single gene mutations (KO) of thrombospondin-1 (TSP-1), thrombospondin-2 (TSP-2), osteopontin (OPN), hevin, and secreted protein acidic rich in cysteine (SPARC) were imaged at six weeks using optical coherence tomography (Stratus, Zeiss) to determine CCT. IOP was measured between 11am and 3pm using TonoLab, one week later. For all measurements, mice were anesthetized using intraperitoneal injection ketamine:xylazine. CCT and IOP were measured in 583 mice (TSP-1 n=71 and 41, TSP-2 n=60 and 32, OPN n=81 and 50, hevin n=59 and 76, SPARC n=54 and 59, WT and KO, respectively). Mean CCT was 5–6% lower in three KO strains—TSP-1, OPN, and SPARC—compared to their corresponding WT (p=1.55×10−7, 1.63×10−11, and 1.91×10−7, respectively). The mean IOP was 8.3%, 6.6%, and 15.1% lower in three KO strains—TSP-1, TSP-2, and SPARC—compared to corresponding WT (p=2.11×10−5, 2.93×10−3, and 3.76×10−9, respectively. Linear regression of IOP versus CCT yielded no statistically significant within-strain correlations for TSP-1 (p=0.12 and 0.073), TSP-2 (p=0.473 and 0.92), OPN (p=0.212 and 0.916), Hevin (p=0.746 and 0.257), and SPARC (p=0.080 and 0.056), reported as p-values considering a null hypothesis of zero slope (WT and KO, respectively). Neither C57-derived strains (TSP-1 and OPN) nor 129-derived strains (TSP-2, hevin, SPARC) demonstrated a correlation between mean IOP and mean CCT across different strains (p=0.75 and p=0.53, respectively). Taken together, these results indicate that CCT is not required to interpret TonoLab IOP readings in the mice when CCT varies 10% about the mean. This does not exclude the possibility of an IOP-CCT correlation for CCT values outside this range or for inter-strain comparisons where the mean CCT differs more than 10%.
Keywords: TonoLab, Transgenic Mice, Glaucoma, Trabecular meshwork, Central corneal thickness, Intraocular pressure, Rebound tonometry
1. Introduction
Glaucoma is one of the leading causes of blindness worldwide and increased intraocular pressure (IOP) is thought to contribute to the progression of the most common form, primary open angle glaucoma (POAG) (Nemesure et al., 2007). Mouse models have been used in the study of IOP regulation (Anderson et al., 2001; John et al., 1999; Savinova et al., 2001) and have been made more accessible owing to recent advances in IOP measurement tools (Danias et al., 2003; Iliev et al., 2006; Kontiola et al., 2001). In mice, IOP varies widely within a mean normal range of 10 to 20-mmHg and can be affected by factors such as age, obesity, and glycemic control (Savinova et al., 2001). Measurement tools, however, have become increasingly reliable, noninvasive, and operator independent with lower variability in measurements (Kontiola et al., 2001).
Prior to the development of rebound tonometry (RT), other techniques to measure IOP were used such as microneedle catheterization of the eye (John et al., 1997) and a modified Goldman applanation tonometry (GAT), which involves the reduction of biprism angles in the instruments applanating tips, was the most widely used and closely studied method for IOP measurement in mice (Cohan and Bohr. 2001; Kim et al., 2007). However, GAT is highly operator dependent and has an observed decline in IOP readings with repeated measurements as is often performed in research studies (Dohadwala et al., 1998).
Based on the induction-impact model, rebound tonometry (RT) rapidly bounces a magnetized probe off of the central cornea and analyzes the return motion with a sensing coil (Danias et al., 2003). The inverse of deceleration time correlates strongly with IOP. Readings do not differ significantly as long as the probe is placed at a starting distance between 3 and 5mm and at an angle of impact between 0 and 25 degrees (Kontiola et al., 2001). RT has improved correlation with invasive manometry readings over GAT and is less affected by corneal factors such as central corneal thickness (CCT), which is particularly important in mice given the increased fraction of applanated area to the total corneal surface (Kim et al., 2007). Compared to other methods, such as the TonoPen which overestimates manometric IOP at low values and underestimates at high values, RT has greater accuracy and less variability in rats (Nissirios et al., 2007), normal mice, and mice with experimental glaucoma (Pease et al., 2011; Pease et al., 2006).
In humans, pneumotonometry, GAT, and TonoPen all have a positive correlation between CCT and measured IOP (Bhan et al., 2002). The RT-based iCare has also shown a weak positive correlation with CCT in humans (Iliev et al., 2006), which has raised the question of whether CCT is a factor to consider when using RT in the mouse model. One study of five commonly used mouse strains found significant differences in mean IOP, but found no correlation between the IOP, CCT or other calibration constants (Nissirios et al., 2007). This suggests that, in the mouse strains studied, CCT is not the primary factor influencing calibration curves.
The matricellular family of proteins consists of nearly ubiquitous, secreted, non-structural proteins that help cells regulate their surrounding extracellular matrix. Some, but not all, mouse strains with single gene mutations of matricellular proteins have demonstrated an alteration in IOP implying a possible regulatory role in IOP (Haddadin et al., 2009; Kang et al., 2011; Chowdhury et al., 2011; Keller et al., Submitted). Because matricellular proteins can affect extracellular matrix (ECM), it is possible that the transgenic mutations could cause a significant change in CCT. We hypothesized that CCT and IOP would correlate both within and across strains. Additionally, we hypothesized that the CCT would differ between the transgenic knockout mice and their wild-type counterparts.
2. Materials and methods
2.1 Animal Care and Husbandry
All experiments were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and received IRB approval from the Massachusetts Eye and Ear Infirmary (MEEI). Several wild-type (WT) and transgenic mouse strains with single gene mutations (KO) of thrombospondin-1 (TSP-1), thrombospondin-2 (TSP-2), osteopontin (OPN), hevin, and secreted protein acidic rich in cysteine (SPARC) were studied (Table 1). Of note, the official Mouse Genome Informatics (MGI) symbols for the affected genes in TSP-1, TSP-2, OPN, and hevin strains are Thbs1, Thbs2, Optn, and Sparcl1 (SPARC-like 1), respectively. However, for the sake of clarity and consistency with previous publications, the above abbreviated mouse strain names are used throughout. TSP-1 KO mice and their WT strain, TSP-2 KO mice and their WT strain, and OPN KO and their WT strain were all purchased from Jackson Laboratory ies (Bar Harbor, Maine). Hevin KO mice and their WT strain were provided as a generous gift from Renata Pasqualini (MD Anderson Cancer Center, University of Texas, Houston). SPARC KO mice and their WT strain were initially obtained from the Benaroya Research Institute at Virginia Mason (Seattle, WA) (Haddadin et al., 2009). All mice were bred at the MEEI animal facility, fed ad libitum, and housed at 21°C in clear plastic rodent cages under 12-hour light/12-hour dark cycles (on 07:00, off 19:00). In order to prevent species drift, heterozygotes were bred and all progeny were subsequently genotyped to separate and create the WT and KO populations for eventual study. All measurements were taken on mice between 5 and 8 weeks of age. The mouse iridocorneal angle and its structures reach maturity by 5 weeks (Smith et al., 2001).
Table 1.
Mouse strain genetic backgrounds
| Strain | Genotype | Genetic background | Type | Generation* | Origin | Reference |
|---|---|---|---|---|---|---|
| TSP-1 | WT | C57BL/6J | Inbred | - | Jackson Lab | (Lawler et al. 1998) |
| KO | B6;129S4-Thbs2tm1Bst/J | Congenic | N8 + F13 | |||
| TSP-2 | WT | B6129SF2/J | F2 hybrid | - | Jackson Lab | (Agah et al. 2002) |
| KO | B6;129S4-Thbs2tm1Bst/J | Targeted mutation | F? + 3PN1 | |||
| OPN | WT | C57BL/6J | Inbred | - | Jackson Lab | (Liaw et al. 1998) |
| KO | B6;129S-Sparctm1Hwe/J | Congenic | N10 + N2F5 | |||
| Hevin | WT | B6129SF2/J | F2 hybrid | - | MD Anderson | (Kang et al. 2011) |
| KO | B6;129S6(Cg)-Spp1tm1Blh/J | Congenic | N10 | |||
| SPARC | WT | B6129SF2/J | F2 hybrid | - | EH Sage Lab | (Norose et al. 1998) |
| KO | B6;129S2-Thbs1tm1Hyn/J | Targeted mutation | - | |||
the number of generations was provided by Jackson Laboratory.
N: the number of backcross generations.
F: the number of inbreeding generations.
P: the number of generations when a strain was cryopreserved.
?: Unknown number of generations when mouse was obtained.
2.2 Optical Coherence Tomography
Eyes of adult mice (at 6 weeks) were imaged using optical coherence tomography (OCT) (Stratus; Carl Zeiss Meditec Inc.; Dublin, CA). Under general anesthesia by intraperitoneal (IP) injection of a ketamine/xylazine mixture (100 mg/kg and 9 mg/kg, respectively; Phoenix Pharmaceutica, St. Joseph, MO), mouse eyes were scanned to acquire images that were analyzed using the OCT software (Stratus version 4.0.7; Carl Zeiss Meditec). CCT was determined by measuring the distance between 2 peaks representing the corneal epithelium and endothelium. Measurements were performed in triplicate for each eye by the same investigator who was masked to the mouse strain. Values were averaged and reported as means and standard deviations. We have previously validated the use of OCT in mice to estimate CCT against high-frequency ultrasound and histology (Haddadin et al., 2009). As previously reported in Haddadin et al., 2009, we generally study mice that are younger than 8 weeks to avoid the potential confounding effects of cataracts, such as phacomorphic or phacolytic glaucoma. These conditions may occur in mature cataracts. SPARC KO mice, for example, have been shown to develop immature cataracts after 1.5 months and mature cataracts without complications between 3.5 and 4.5 months; some cataracts, however, cause complications after 5 months of age (Norose et al., 1998).
2.3 Measurement of IOP
Mouse IOP was measured as previously described (Haddadin et al., 2009). Briefly, mice were anesthetized by intraperitoneal (IP) injection of a ketamine/xylazine. Per manufacturer recommendations, the rebound tonometer (TonoLab, Colonial Medical Supply, Franconia, NH) was fixed horizontally to allow perpendicular contact with the central cornea, and the tip of the probe was positioned between 2 and 3 mm from the eye. To reduce variability, the rebound tonometer was modified to include a pedal that activated the probe, obviating handling of the device. Target verification was performed under direct visualization at 5.5 × magnification. A single measurement was accepted only if the device indicated that there was “no significant variability” (per the protocol manual; Colonial Medical Supply). The average IOP was taken from three sets of six measurements of IOP in each eye, alternating right and left eye, with the starting eye picked at random (Wang et al., 2005; Saeki et al., 2008). All measurements were taken between 4 and 7 minutes after IP injection, as previous studies have shown this to be a period of stable IOP (Savinova et al., 2001; Aihara et al., 2003). Previous studies (Danias et al., 2003) have shown that weekly administration of this anesthesia mixture (ketamine/xylazine) does not affect IOP. IOP was measured once per mouse, between 11 am and 3 pm at 7 weeks of age—1 week after CCT measurement. Furthermore, as previously reported, we have performed manometric validations of TonoLab IOP measurement in several mouse strains (Haddadin et al., 2009; Kang et al., 2011). Briefly, mouse eyes were cannulated through the temporal limbus with a 30-gauge needle attached to a water reservoir and pressure transducer as described elsewhere (Danias et al., 2003). IOP measurements were taken at various reservoir heights between 7 and 37 mm Hg in random ascending and descending order with the open-stopcock technique, and linear regressions of water reservoir height (mmHg) versus TonoLab IOP (mmHg) confirmed strong correlation (r2>0.99).
2.4 Statistical Analysis
All CCT and IOP data were presented as mean ± SD. In order to achieve 90% power to detect a 5% difference in CCT (roughly 5µm), a combined sample size of 44 is required. In order to achieve 90% power to detect a 10% difference in IOP (roughly 1.7 mmHg), a combined sample size of 60 is required. Differences in means were tested using a two-tailed student t-test with p <0.05 considered a statistically significant difference. Since only pairwise comparisons are reported (i.e. WT vs. KO in each case) we elected not to correct for multiple comparisons. Linear regression equations were calculated after plotting IOP versus CCT within strains and mean IOP versus mean CCT across strains and p < 0.05 was considered a statistically significant deviation from a null hypothesis of zero slope. Detecting an r2 of 0.10 or greater with 90% power requires n > 79 (Faul et al., 2009).
3. Results
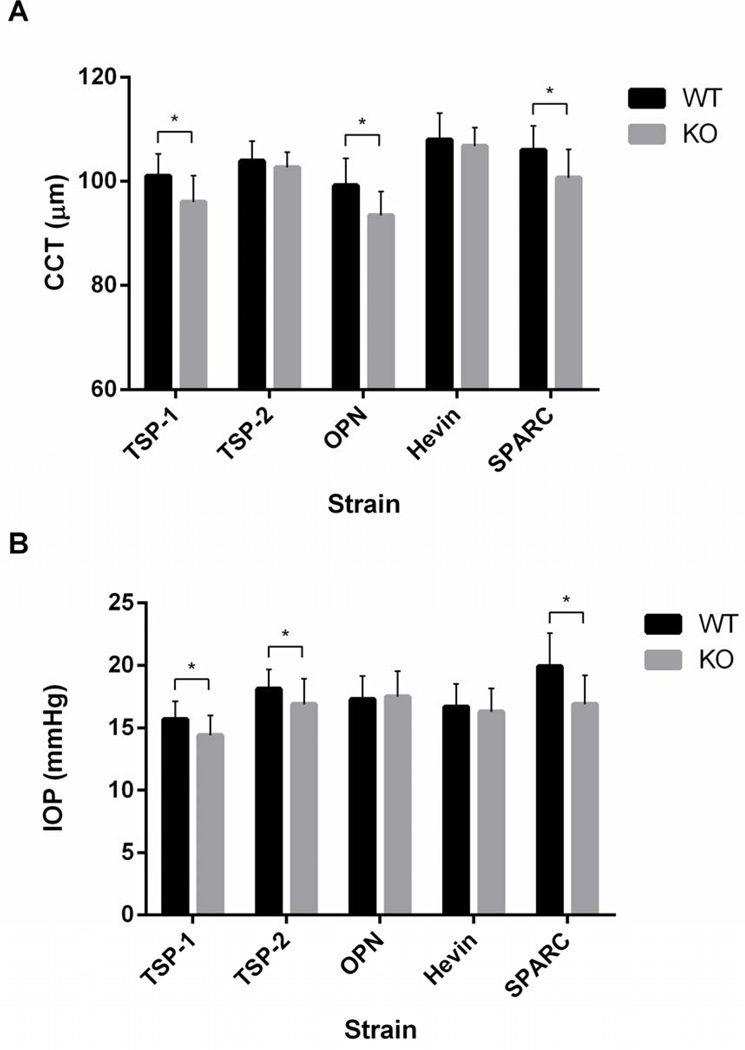
CCT and IOP were measured in 583 mice (TSP-1 n = 71 and 41, TSP-2 n = 60 and 32, OPN n = 81 and 50, Hevin n = 59 and 76, SPARC n = 54 and 59, for WT and KO, respectively). Mean CCT ranged from 93.4 ± 4.6 to 108.0 ± 5.1 µm in the different strains (Fig. 1A) and was 5–6% lower in three KO strains—TSP-1, OPN, and SPARC—compared to their WT counterparts (p < 0.0001 for all three) (Table 2). Mean strain IOP ranged from 14.4 ± 1.6 to 19.9 ± 2.7 mmHg (Fig. 1B) and was 8.3%, 6.6%, and 15.1% lower in three KO strains—TSP-1, TSP-2, and SPARC—compared to their WT counterparts (p < 0.01 in all cases) (Table 3), confirming previously reported IOP differences in these strains (Haddadin et al., 2009; Haddadin et al., 2012).
Figure 1.
Mean (A) CCT and (B) TonoLab-obtained IOP in sedated mice of five matricellular KO strains and WT counterparts. Error bars represent standard deviation (SD). Asterisks indicate a statistically significant difference (p < 0.05) by student t-test.
Table 2.
Mouse CCT ± SEM
| Strain | WT (n) | KO (n) | % difference | p-value |
|---|---|---|---|---|
| TSP-1 | 101.0 ± 0.51 (72) | 96 ± 0.79 (41) | −4.95* | <0.0001 |
| TSP-2 | 103.9 ± 0.50 (60) | 102.7 ± 0.52 (32) | −1.15 | 0.13 |
| OPN | 99.2 ± 0.58 (81) | 93.4 ± 0.66 (49) | −5.85* | <0.0001 |
| Hevin | 108.0 ± 0.80 (59) | 106.8 ± 0.51 (76) | −1.11 | 0.19 |
| SPARC | 106.0 ± 0.68 (55) | 100.7 ± 0.45 (59) | −5.00* | <0.0001 |
Table 3.
Mouse IOP ± SEM
| Strain | WT (n) | KO (n) | % difference | p-value |
|---|---|---|---|---|
| TSP-1 | 15.7 ± 0.17 (72) | 14.4 ± 0.25 (41) | −8.28* | <0.0001 |
| TSP-2 | 18.1 ± 0.20 (60) | 16.9 ± 0.36 (32) | −6.63* | <0.0001 |
| OPN | 17.3 ± 0.21 (81) | 17.5 ± 0.29 (49) | +1.16 | 0.57 |
| Hevin | 16.7 ± 0.29 (59) | 16.3 ± 0.22 (76) | −2.40 | 0.26 |
| SPARC | 19.9 ± 0.25 (55) | 16.9 ± 0.24 (59) | −15.08* | <0.0001 |
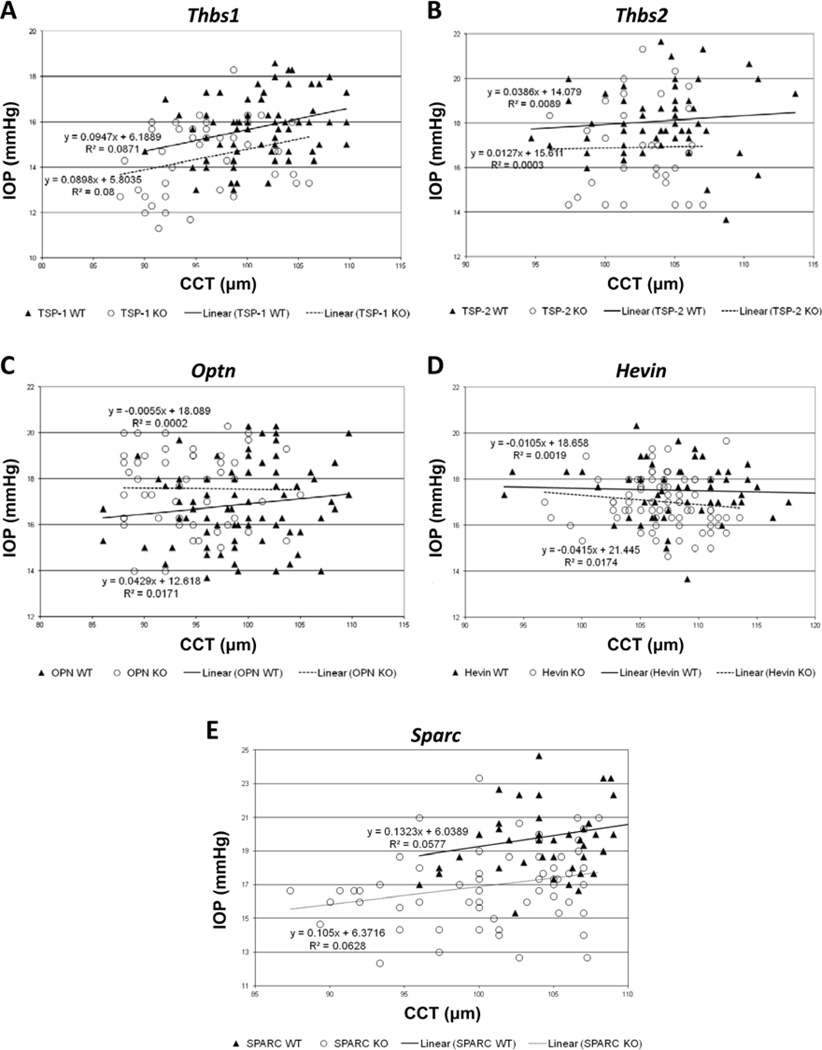
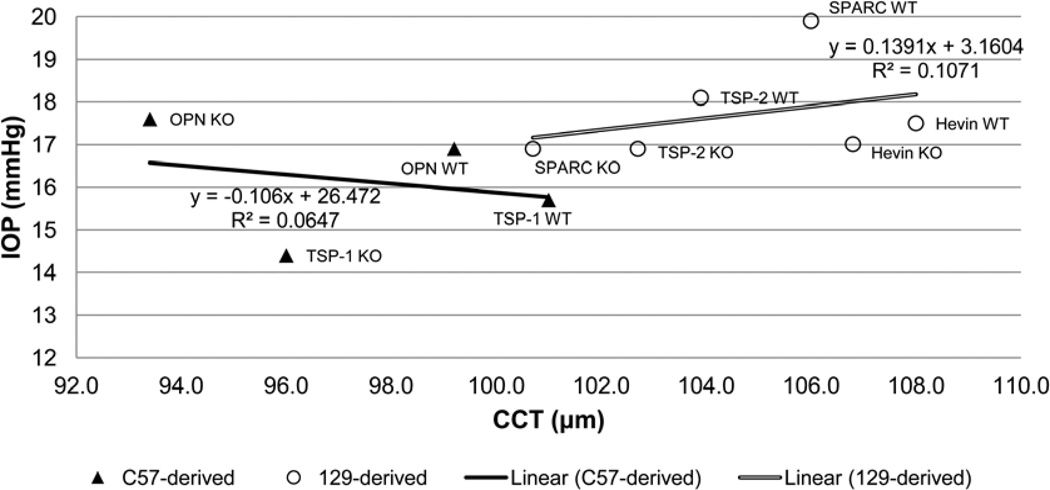
Linear regressions calculated from scatter plots of IOP versus CCT in each strain yielded no statistically significant correlations, with the null hypothesis of zero slope (Fig. 2A–E). Neither C57-derived mice nor 129-derived mice demonstrated a statistically significant correlation between mean IOP and mean CCT across strains (Fig. 3). As previously observed, 129-derived strains tended to have both higher CCT and IOP than C57-derived strains (Fig. 3) (Chowdhury et al., 2011; Haddadin et al., 2009; Haddadin et al., 2012; Kang et al., 2011).
Figure 2.
Correlation of TonoLab-obtained IOP values with CCT within five strains of WT mice and their matricellular KO counterparts, respectively. (A) p = 0.12 and 0.073 [n = 71 and 41], (B) p = 0.47 and 0.92 [n = 60 and 32], (C) p = 0.21 and 0.92 [n = 81 and 50], (D) p = 0.75 and 0.26 [n = 59 and 76], and (E) p = 0.080 and 0.056 [n = 54 and 59]. In each case, the mutated mouse gene is displayed at the top of the panel.
Figure 3.
Correlation of mean TonoLab-obtained IOP with mean CCT across C57- and 129-derived mouse strains. (p = 0.75 and 0.53, respectively).
4. Discussion
Differences in both mean CCT and mean IOP can be observed across mouse species. We have previously performed manometric validations of TonoLab IOP measurement in SPARC and hevin KO and corresponding WT mouse strains (Haddadin et al., 2009; Kang et al., 2011). The results of this study confirm our previous reports demonstrating a lower IOP in SPARC, TSP-1 and TSP-2 KO mice compared to their corresponding WT mice (Tables 3 and 4), while transgenic mutations of hevin and OPN do not appear to alter the IOP (Chowdhury et al., 2011; Haddadin et al., 2009; Haddadin et al., 2012; Kang et al., 2011). We also confirmed the thinner CCT in TSP-1 KO mice (Tables 2 and 5); and similarly did not find a difference in CCT of TSP-2 or hevin KO mice. Different from our previous report, we found a thinner CCT in SPARC KO mice.
Table 4.
Previously published mouse IOP ± SEM
| Strain | WT (n) | KO (n) | % difference | p-value |
|---|---|---|---|---|
| TSP-1 | 15.8 ± 0.18 (68) | 14.2 ± 0.24 (70) | −10.13* | <0.0001 |
| TSP-2 | 18.1 ± 0.22 (54) | 16.8 ± 0.27 (56) | −7.18* | <0.0001 |
| OPN | 17.3 ± 0.23 (68) | 17.5 ± 0.27 (56) | +1.16 | 0.57 |
| Hevin | 15.9 ± 0.28 (46) | 15.3 ± 0.30 (44) | −3.77 | 0.15 |
| SPARC | 19.9 ± 0.28 (104) | 16.9 ± 0.20 (142) | −15.07* | <0.0001 |
Table 5.
Previously published mouse CCT ± SEM
| Strain | WT (n) | KO (n) | % difference | p-value |
|---|---|---|---|---|
| TSP-1 | 101.1 ± 0.47 (92) | 94.5 ± 0.73 (57) | −6.53%* | <0.0001 |
| TSP-2 | 103.9 ± 0.47 (60) | 102.7 ± 0.51 (32) | −1.15% | 0.12 |
| OPN | 99.2 ± 0.66 (70) | 91.7 ± 0.51 (50) | −7.56%* | <0.0001 |
| Hevin | 107.9 ± 0.75 (44) | 106.8 ± 0.54 (42) | −1.02% | 0.11 |
| SPARC | 105.6 ± 1.15 (14) | 104.5 ± 1.59 (6) | −1.04% | 0.60 |
In this study, we measured 114 SPARC KO and WT mice compared to 20 in our first report. In our initial report, we found that SPARC KO mice had a 1% thinner CCT (not statistically significant). With the much larger sample, we now find SPARC KO mice as having a 5% thinner CCT which achieved statistical significance. While the much larger sample size allows for greater statistical power to differentiate small differences, there is an increased chance of a type 2 statistical error (i.e. obtaining a statistically significant result where no difference is truly present). We do not believe that this statistically significant difference in CCT has biologic significance. In our initial report, we performed manometric validation of the TonoLab indicating that its measurements, in those strains of mice, were not subject to artifact of CCT as applanation tonometry can be in humans. Furthermore, the regression analyses presented in this report show no CCT-IOP correlations for any of the species studied.
While we have found statistical differences in CCTs, the small magnitude of the difference indicates that experimental technique variability could have a significant effect. For example, technique variation of OCT measurement could be relevant. Any angle away from exactly 90 degrees of incident beam (i.e. an oblique cut) could artifactually thicken the cornea. However, the measurement line across the cornea can be directly observed during the time of measurement. The resolution of time-domain OCT is 10 microns (Forooghian et al., 2008). Even though every effort is made to keep the cornea hydrated, differences in corneal hydration could result in thinner corneas if there is dehydration. Every effort is made to image the central cornea, but the cornea begins to change as measurements deviate away from the center. We believe that all of the potential experimental confounders were minimized due to the small standard deviations.
Our findings here suggest that CCT does not affect TonoLab IOP readings within strains and are not needed to interpret IOP measurements in mice when CCT varies by no more than 10% about the mean (representing roughly 10 µm, or 2 standard deviations, in the strains studied). Assuming a confidence level of 0.05, the study had 90% power to detect a 0.10 coefficient of determination (r2), suggesting that it is unlikely that we would fail to detect a correlation in which at least 10% of the change in IOP is explained by CCT alone. Our results do not, however, exclude the possibility of an effect on TonoLab IOP readings for CCT values outside of this range. For extremely thick or extremely thin corneas, RT-based measurements may still be affected in mice.
The current study failed to find evidence that measurement of IOP in closely-related mouse strains can be correlated to CCT. Across-strain regression in the two subgroups—C57-derived and 129-derived—demonstrated no statistically significant correlation between mean IOP and mean CCT. Study of four different C57-derived strains and six different 129-derived strains resulted in 7% and 10% power, respectively, to detect an r2 of 0.10 or greater. In both groups of strain derivatives, mean strain CCT deviated at most by 5% (or roughly 6 µm) from the overall group mean. While we observed a 5–6% decrease in CCT in TSP-1, OPN, and SPARC KO mice, we detected no statistically significant correlation with IOP across the several strains studied. These relatively small variations in CCT are likely detected due to exceedingly precise measurements and are unlikely to affect IOP. We cannot exclude the possibility that TonoLab IOP measurements vary with CCT across strains that differ more than 10% in mean CCT. Additionally, our results are not generalizable beyond the strains included in this study; for example, DBA/2J mice are prone to corneal opacities and it is unclear if the findings of the current study would extend to strains beyond B6 and 129.
Our results were similar to Nissirios et al who examined the relationship between CCT and TonoLab IOP in mice (Nissirios et al., 2007). Unlike the previous study, the mice in this study were sedated during IOP measurement, indicating the lack of effect of anesthesia as an experimental variable so long as all mice are consistently measured awake or sedated. Our study included multiple transgenic species used in the study of POAG that have demonstrated differential expression of matricellular proteins in the trabecular meshwork—such as OPN, Hevin, and SPARC—and show a broader range of mean IOPs (Rhee et al., 2003; Kang et al., 2011; Chowdhury et al., 2011; Rhee et al., 2009) . Also, while Nissirios et al limited their linear regression analyses to eight male mice per strain, our study included both male and female mice, with sufficient power to detect even a smaller degree of within-strain CCT-IOP correlation.
In the future, it may be important to study the effects of other corneal properties, such as curvature, elasticity, and rigidity on RT-based mouse IOP measurements (Moreno-Montanes et al., 2011), as these properties are not reflected in simple CCT measurement (Munger et al., 2001) and may also have an impact on probe head deceleration times. Additionally, studying mice at older ages may be needed as some have reported that CCT may not be fully matured until 7–8 weeks for C57BL/6 mice (Burns et al., 2011). For now, the evidence suggests no systemic influence of CCT on the ability of RT-based instruments to measure IOP (Danias et al., 2003; Iliev et al., 2006).
4.1 Conclusion
Taken together, these data suggest that—in the matricellular transgenic mouse strains studied—CCT need not be measured to interpret TonoLab-obtained IOP, but this does not exclude the possibility of an IOP-CCT correlation in other species or when CCT falls outside of this range. These data also confirm previous reports of IOP differences and reveal a new CCT difference, but demonstrate no correlation between CCT and TonoLab-obtained IOP measurements in these strains.
Highlights.
• With increased sample size, we now find slightly thinner CCT in SPARC KO mice.
• We find no correlation between CCT and TonoLab-obtained IOP within these strains.
• We confirm previous reports showing lower IOP in SPARC, TSP-1 and TSP-2 KO mice.
• We confirm previous reports showing thinner CCT in TSP-1 KO mice.
ACKNOWLEDGEMENTS
FUNDING:
Supported by Howard Hughes Medical Institute Student Research Fellowship, Massachusetts Lions Research Foundation, NIH R01 EY 019654-01 (DJR), and NIH EY 014104 (MEEI Vision-Core Grant)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am. J. Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp. Eye Res. 2003;77:681–686. doi: 10.1016/j.exer.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Savinova OV, Hawes NL, Chang B, Zabaleta A, Wilpan R, Heckenlively JR, Davisson M, John SW. Genetic modification of glaucoma associated phenotypes between AKXD-28/Ty and DBA/2J mice. BMC Genet. 2001;2:1. doi: 10.1186/1471-2156-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A, Browning AC, Shah S, Hamilton R, Dave D, Dua HS. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest. Ophthalmol. Vis. Sci. 2002;43:1389–1392. [PubMed] [Google Scholar]
- Chowdhury UR, Jea SY, Oh DJ, Rhee DJ, Fautsch MP. Expression profile of the matricellular protein osteopontin in primary open-angle glaucoma and the normal human eye. Invest. Ophthalmol. Vis. Sci. 2011;52:6443–6451. doi: 10.1167/iovs.11-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan BE, Bohr DF. Measurement of intraocular pressure in awake mice. Invest. Ophthalmol. Vis. Sci. 2001;42:2560–2562. [PubMed] [Google Scholar]
- Danias J, Kontiola AI, Filippopoulos T, Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest. Ophthalmol. Vis. Sci. 2003;44:1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- Dohadwala AA, Munger R, Damji KF. Positive correlation between Tono-Pen intraocular pressure and central corneal thickness. Ophthalmology. 1998;105:1849–1854. doi: 10.1016/S0161-6420(98)91029-6. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest. Ophthalmol. Vis. Sci. 2008;49:4290–4296. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadin RI, Oh DJ, Kang MH, Filippopoulos T, Gupta M, Hart L, Sage EH, Rhee DJ. SPARC-null mice exhibit lower intraocular pressures. Invest. Ophthalmol. Vis. Sci. 2009;50:3771–3777. doi: 10.1167/iovs.08-2489. [DOI] [PubMed] [Google Scholar]
- Haddadin RI, Oh DJ, Kang MH, Villarreal G, Jr, Kang JH, Jin R, Gong H, Rhee DJ. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest. Ophthalmol. Vis. Sci. 2012;53:6708–6717. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon SD, Patel NB, Burns AR. Assessment of postnatal corneal development in the C57BL/6 mouse using spectral domain optical coherence tomography and microwave-assisted histology. Exp. Eye Res. 2011;93:363–370. doi: 10.1016/j.exer.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ME, Goldblum D, Katsoulis K, Amstutz C, Frueh B. Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Br. J. Ophthalmol. 2006;90:833–835. doi: 10.1136/bjo.2005.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SW, Anderson MG, Smith RS. Mouse genetics: a tool to help unlock the mechanisms of glaucoma. J. Glaucoma. 1999;8:400–412. [PubMed] [Google Scholar]
- John SW, Hagaman JR, MacTaggart TE, Peng L, Smithes O. Intraocular pressure in inbred mouse strains. Invest. Ophthalmol. Vis. Sci. 1997;38:249–253. [PubMed] [Google Scholar]
- Kang MH, Oh DJ, Rhee DJ. Effect of hevin deletion in mice and characterization in trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2011;52:2187–2193. doi: 10.1167/iovs.10-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Vranka J, Haddadin R, Kang MH, Oh DJ, Rhee DJ, Yang YF, Sun Y, Kelley MJ, Acott TS. The effects of tenascin C knockdown on trabecular meshwork outflow resistance. Iovs. doi: 10.1167/iovs.13-11620. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CY, Kuehn MH, Anderson MG, Kwon YH. Intraocular pressure measurement in mice: a comparison between Goldmann and rebound tonometry. Eye (Lond) 2007;21:1202–1209. doi: 10.1038/sj.eye.6702576. [DOI] [PubMed] [Google Scholar]
- Kontiola AI, Goldblum D, Mittag T, Danias J. The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp. Eye Res. 2001;73:781–785. doi: 10.1006/exer.2001.1088. [DOI] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J. Clin. Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J. Clin. Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Montanes J, Gosende I, Caire J, Garcia-Granero M. Comparation of the new rebound tonometer IOPen and the Goldmann tonometer, and their relationship to corneal properties. Eye (Lond) 2011;25:50–56. doi: 10.1038/eye.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger R, Dohadwala AA, Hodge WG, Jackson WB, Mintsioulis G, Damji KF. Changes in measured intraocular pressure after hyperopic photorefractive keratectomy. J. Cataract Refract. Surg. 2001;27:1254–1262. doi: 10.1016/s0886-3350(01)00971-3. [DOI] [PubMed] [Google Scholar]
- Nemesure B, Honkanen R, Hennis A, Wu SY, Leske MC Barbados Eye Studies Group. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114:1810–1815. doi: 10.1016/j.ophtha.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Nissirios N, Goldblum D, Rohrer K, Mittag T, Danias J. Noninvasive determination of intraocular pressure (IOP) in nonsedated mice of 5 different inbred strains. J. Glaucoma. 2007;16:57–61. doi: 10.1097/IJG.0b013e31802b3547. [DOI] [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest. Ophthalmol. Vis. Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- Pease ME, Cone FE, Gelman S, Son JL, Quigley HA. Calibration of the TonoLab tonometer in mice with spontaneous or experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2011;52:858–864. doi: 10.1167/iovs.10-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease ME, Hammond JC, Quigley HA. Manometric calibration and comparison of TonoLab and TonoPen tonometers in rats with experimental glaucoma and in normal mice. J. Glaucoma. 2006;15:512–519. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- Rhee DJ, Fariss RN, Brekken R, Sage EH, Russell P. The matricellular protein SPARC is expressed in human trabecular meshwork. Exp. Eye Res. 2003;77:601–607. doi: 10.1016/s0014-4835(03)00190-8. [DOI] [PubMed] [Google Scholar]
- Rhee DJ, Haddadin RI, Kang MH, Oh DJ. Matricellular proteins in the trabecular meshwork. Exp. Eye Res. 2009;88:694–703. doi: 10.1016/j.exer.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Saeki T, Aihara M, Ohashi M, Araie M. The efficacy of TonoLab in detecting physiological and pharmacological changes of mouse intraocular pressure--comparison with TonoPen and microneedle manometery. Curr. Eye Res. 2008;33:247–252. doi: 10.1080/02713680801919716. [DOI] [PubMed] [Google Scholar]
- Savinova OV, Sugiyama F, Martin JE, Tomarev SI, Paigen BJ, Smith RS, John SW. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2:12. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev. Biol. 2001;1:3. doi: 10.1186/1471-213X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest. Ophthalmol. Vis. Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]