Abstract
Background
This analysis assessed the acquisition (incidence) and persistence (clearance) of HPV infection by self-reported race among men in The HPV in Men (HIM) Study, a multinational prospective study of the natural history of genital HPV infections.
Methods
Self-reported race was categorized as White, Black, Asian/Pacific Islander (PI), or multiple and mixed race. Genital samples were combined for HPV DNA testing and categorized by any-, oncogenic-, and non-oncogenic HPV infections.
Results
Asian/PI race had significantly the lowest incidence of any-, oncogenic-, and non-oncogenic HPV infection (P < 0.001). In multivariable analyses Asian/PI race was associated with a lower probability of acquiring any- (HR=0.63; 95% CI 0.42–0.95) and non-oncogenic HPV infection (HR=0.61; 95% CI 0.40–0.93) when compared to Whites. No significant associations were evident for Asian/PI race for clearance. Multiple and mixed race was significantly associated with lower probability of acquiring non-oncogenic HPV infection (HR=0.83; 95% CI 0.69–0.99) and borderline significant associations were observed for any HPV (HR=0.91) and oncogenic infections (HR=0.92). Multiple and mixed race was associated with a lower probability of clearing any- (HR=0.92; 95% CI 0.84–1.00) and oncogenic HPV infections (HR=0.85; 95% CI 0.75–0.95).
Conclusion
Asian/PI race had the lowest incidence of HPV and exhibited a lower probability of acquiring new HPV infections. Multiple and mixed race had the second lowest incidence of infection and was associated with a lower probability of acquiring and clearing a HPV infection.
Impact
Race-specific differences in HPV infection could be due to behavior, innate genetic differences, or circulating intratypic HPV variants.
INTRODUCTION
Human papillomavirus (HPV) is an established human carcinogen capable of infecting and causing cancer at multiple anatomical sites. HPV infection is highly prevalent among both men and women [1, 2]. With >100 types identified, HPVs are divided into low-risk (e.g., HPVs 6, 11, 42, 43, and 44), that have the potential to induce warts and low-grade squamous intraepithelial lesions, and high-risk (e.g., HPVs 16, 18, 31, 33, 35, 45, 51, 52 and 56) that are causally involved in cervical cancer and cancers at other anatomical sites [3]. For decades we have known that HPV causes cancer in women, but only recently have we recognized that HPV also causes cancer in men (penis, anogenital, and oropharyngeal) [4], cancers that occur at older ages in men compared to women and cancers for which we have no reliable screening tools. However, most HPV infections are transient and asymptomatic or subclinical, do not result in disease, and are usually self-cleared. Although the quadrivalent HPV vaccine is available for males and females ages 9 to 26 [5], immunization rates in the US are low, and the vaccine is not available to those ≥ 27 years. Revealing risk factors that may have a role in explaining variations in incidence and persistence of HPV infection may reveal new underpinnings of this infection that can lead to strategies to reduce HPV-related disease.
Previous studies have provided widely varied estimates of HPV prevalence in men ranging from 0% to 73% [1]. Prevalence of genital HPV infection in men appears to vary by world region and to date there is little information on the acquisition (incidence) and persistence (clearance) of HPV infection in men by race. In a previous report [6] we assessed prevalence of HPV infection by race and observed the lowest HPV prevalence among Asian/Pacific Islanders (PI) even after adjusting for potential confounding factors. Our previous findings were consistent with previous studies that reported a lower prevalence of HPV infection among Asians in Continental Asia [7, 8]. Presently it is unknown why Asian/PI race possesses a lower prevalence of HPV infection but these differences could be attributed to one or more factors including behavioral, socioeconomic, geographic distributions of HPV types, innate genetic differences, or circulating intratypic HPV variants. Building on our previous race-specific HPV prevalence data, the purpose of this analysis was to assess the influence of self-reported race on the incidence and clearance of HPV infections among men in The HPV in Men (HIM) Study. To our knowledge this is the first study to assess the incidence and clearance of genital HPV natural history by race among men.
MATERIALS AND METHODS
Study Population
The HIM study is an on-going prospective, multinational study of the natural history of HPV infection in men [9, 10]. Study inclusion criteria included ages 18 to 70 years and residents of southern Florida in USA, São Paulo in Brazil, or Cuernavaca in Mexico with no plans to relocate within the next 4 years from baseline. Participants also reported no previous diagnosis of genital or anal warts, had not participated in an HPV vaccine study, reported no previous diagnosis of HIV, reported no current penile discharge or burning sensation during urination, were not being treated for sexually transmitted infections, had not been incarcerated during the preceding 6 months, had not been in drug treatment during the preceding 6 months, and were willing to comply with ten scheduled visits every 6 months for 4 years. The total sample size for this analysis with complete available data was 3,973 men. All study subjects in this analysis completed at least two visits. The median number of clinic visits completed was four visits and the median interval between visits 6.23 months.
Men were recruited in three age groups (18 to 30 years, 31 to 44 years, and 45 to 70 years). In Mexico, men were recruited through a large health plan, from factories and military in Cuernavaca and Morelos. In Brazil, men were recruited through television, radio, and newspaper advertisement, and from a large clinic in São Paulo providing genitourinary services: including tests for HIV and sexually transmitted infections. In the USA, men were recruited mainly from the University of South Florida and the general community in Tampa, Florida. A full description of cohort procedures, HPV prevalence, and factors associated with prevalent infections has already been reported [9, 10]. Men who met all the inclusion criteria reviewed a written informed consent with a trained member who gave the men opportunity to ask questions and decline participation if so desired. The human-subjects’ committees of the Ludwig Institute for Cancer Research, São Paulo, Brazil, The Centro de Referencia e Tratamento de Doencas Sexualmente Transmissiveis e AIDS, São Paulo, Brazil, The University of South Florida, USA, and the National Institute of Public Health of Mexico, Cuernavaca, Mexico, approved all study procedures before study initiation. Men who provided consent had a clinical examination two weeks prior to enrollment visit and every 6 months thereafter. Only men who returned for the enrollment visit were included in this study.
Risk Factor Questionnaire
An extensive 88-item computer-assisted self-interview (CASI) sexual history and health questionnaire were given at enrollment to assess sociodemographic characteristics, and risk factors. The questionnaire required approximately 20 minutes to complete and was written in the region’s primary language (Portuguese, Spanish, or English). Self-reported ethnicity was assessed using one question (Hispanic vs. non-Hispanic). For this analysis self-report race was categorized as White, Black, Asian/Pacific Islander, Mestizo, or other/mixed race. As expected, the majority of the men (> 90%) from the Mexican study site self-identified as ‘Mestizo’, which represents multiple races (namely White and American Indian), as they recognize the predominance of their “mixed” ancestry unique to Mexico. For this analysis, other/mixed race and Mestizo were combined into a single category: Multiple and Mixed Race.
Data and Sample Collection/HPV Penile and Scrotal Sampling
Samples were obtained from the external genitalia at each visit by use of Dacron (Digene, Gaithersburg, MD, USA) swabs prewetted with saline. Three separate samples were obtained: corona of glans penis (1 sample), penile shaft (1 sample), and scrotum (1 sample). The samples placed in 450 μL of Specimen Transport Medium, and then combined into one sample before DNA extraction (described below). The specimens were stored at −70°C until PCR analyses and HPV genotyping was performed. We have previously shown the validity of these three anatomical sites in the assessment of HPV status, and high sampling reproducibility for the detection of HPV DNA by use of this method [11].
DNA Extraction and HPV Genotyping
DNA extraction was conducted with QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) on a robotic system according to the manufacturer’s instructions and DNA was stored at 4°C until use. The extracted DNA samples were tested for the presence of HPV types by amplification of 30 ng of DNA with the PGMY09/11 L1 consensus primer system [12, 13]. HPV genotyping was performed with the linear array method on all samples irrespective of the HPV PCR result (Roche Molecular Diagnostics, Alameda, CA, USA). Only samples that tested positive for beta-globin (99% at enrollment) were judged to be adequate and included in the analysis. Before genotyping, the amplification products were run on 2% agarose gels to visualize a 450 base pair band corresponding to HPV amplification for identification of samples that might have a HPV type other than the 37 types analyzed in the genotyping assay. Samples for which HPV was amplified on PCR but did not hybridize with a specific HPV type during the genotyping assay (e.g., unclassified infections) were classified as HPV negative. Across the ten visits the frequency of these unclassified infections that were classified as HPV negative ranged from 1.25% to 4.0%. The HPV types that were classified as oncogenic were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 [14] and non-oncogenic types were 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39.
Statistical Analysis
Sociodemographic and sexual behavioral characteristics by race were compared using the Monte Carlo estimation of exact Pearson chi-square test for categorical variables. Time for newly acquired HPV infection for each man was estimated by use of the time from study entry to the date of the first detection of HPV DNA. For estimates of any or type-specific HPV incidence, only participants who were free of any or a specific HPV type, respectively, at enrollment were included.
For grouped incidence analyses the analytical unit is an individual, so any HPV incidence was defined as a positive test result for at least one of 37 HPV genotypes, oncogenic HPV incidence was defined as a positive test for at least one oncogenic HPV type, and non-oncogenic was defined as a positive test for at least one non-oncogenic HPV type. For type-specific and grouped HPV incidence analyses, only the first acquired infection will be considered at a given HPV type or group so the analytical unit is an individual. Cumulative risk of HPV incidence was estimated using the Kaplan-Meier method, with the log-rank tests used to identify differences across race groups. Twelve-month HPV incidence was also estimated using the Kaplan-Meier method. The association between race and HPV incidence was also assessed with Cox proportional hazards regression after adjusting other factors.
HPV clearance was defined as a participant testing HPV negative at two consecutive visits after testing positive, excluding infections detected for the first time at a participant’s final visit. Men with HPV infections, regardless the infection status at baseline, were included in the clearance analyses. For grouped clearance the analytical unit is an infection so each individual genotype was considered as a separate infection. Since men could have been infected with multiple types of HPV within a defined group (e.g., HPV16 and HPV18 are both oncogenic), we adjusted for within-subject correlation in all grouped HPV clearance analyses. The median time to HPV clearance among all men with an incident infection was estimated using the clustered Kaplan-Meier method [15] and men whose infections did not clear were censored in the analysis. To model the associations between race and grouped HPV clearance, we employed Cox proportional hazards regression with the robust covariance matrix estimator [16].
Candidate factors we considered in the multi-variable modeling included age (continuous), education, marital status, current smoking status, circumcision, lifetime number of female partners, number of female partners in past 3 to 6 months, lifetime number of male partners, and number of male partners in past 3 to 6 months. The categories of these variables were listed in Table 1. Backward selection methods were used to select variables in the multivariable models. Age, the design variable, and race were forced to be included in the multivariable models. In addition, as we observed that HPV infection status at baseline had significant impact on HPV clearance, we included this factor in the multivariable models of HPV clearance. We were also interested in evaluating the effect of race on HPV incidence and clearance among those with similar numbers of lifetime female partners; therefore, stratified analysis by lifetime female partners (0–1, 2–9, and 10+) was conducted. All analyses were conducted with SAS (version 9.3) and tests were two-sided with a significance level of 0.05.
Table 1.
Distribution of sociodemographic and sexual behavior characteristics by race
| Characteristic | Total | White | Black | Asian/PI | Multiple and Mixed Race | P-value1 |
|---|---|---|---|---|---|---|
| Clinic Site | ||||||
| United states | 1306 (32.9%) | 877 (48.6%) | 227 (36.1%) | 89 (80.2%) | 113 (7.9%) | < 0.0001 |
| Brazil | 1383 (34.8%) | 856 (47.4%) | 399 (63.4%) | 22 (19.8%) | 106 (7.4%) | |
| Mexico | 1284 (32.3%) | 72 (4%) | 3 (0.5%) | 0 (0%) | 1209 (84.7%) | |
| Total | 3973 (100%) | 1805 (45.4%) | 629 (15.8%) | 111 (2.8%) | 1428 (35.9%) | |
| Age | ||||||
| 18 to 30 | 1938 (48.8%) | 943 (52.2%) | 279 (44.4%) | 90 (81.1%) | 626 (43.8%) | < 0.0001 |
| 31 to 44 | 1516 (38.2%) | 619 (34.3%) | 256 (40.7%) | 19 (17.1%) | 622 (43.6%) | |
| 45 to 70 | 519 (13.1%) | 243 (13.5%) | 94 (14.9%) | 2 (1.8%) | 180 (12.6%) | |
| Total | 3973 (100%) | 1805 (45.4%) | 629 (15.8%) | 111 (2.8%) | 1428 (35.9%) | |
| Ethnicity | ||||||
| Hispanic | 1781 (45.1%) | 388 (21.7%) | 75 (12.1%) | 5 (4.5%) | 1313 (92.3%) | < 0.0001 |
| Non-Hispanic | 2164 (54.9%) | 1402 (78.3%) | 547 (87.9%) | 106 (95.5%) | 109 (7.7%) | |
| Total | 3945 (100%) | 1790 (45.4%) | 622 (15.8%) | 111 (2.8%) | 1422 (36%) | |
| Years of Education | ||||||
| <1 2 Years | 864 (21.8%) | 216 (12%) | 131 (20.9%) | 1 (0.9%) | 516 (36.3%) | < 0.0001 |
| Completed 12 Years | 1053 (26.6%) | 436 (24.2%) | 233 (37.1%) | 29 (26.4%) | 355 (25%) | |
| 13 to 15 Years | 1019 (25.7%) | 645 (35.8%) | 140 (22.3%) | 52 (47.3%) | 182 (12.8%) | |
| Completed 16 Years | 781 (19.7%) | 380 (21.1%) | 101 (16.1%) | 18 (16.4%) | 282 (19.8%) | |
| ≥ 17 Years | 243 (6.1%) | 124 (6.9%) | 23 (3.7%) | 10 (9.1%) | 86 (6.1%) | |
| Total | 3960 (100%) | 1801 (45.5%) | 628 (15.9%) | 110 (2.8%) | 1421 (35.9%) | |
| Marital Status | ||||||
| Single | 1798 (45.4%) | 1009 (56%) | 298 (47.6%) | 94 (84.7%) | 397 (27.9%) | < 0.0001 |
| Married | 1347 (34%) | 433 (24%) | 158 (25.2%) | 11 (9.9%) | 745 (52.3%) | |
| Cohabiting | 471 (11.9%) | 170 (9.4%) | 95 (15.2%) | 3 (2.7%) | 203 (14.3%) | |
| Divorced, Separated, Widowed | 346 (8.7%) | 189 (10.5%) | 75 (12%) | 3 (2.7%) | 79 (5.5%) | |
| Total | 3962 (100%) | 1801 (45.5%) | 626 (15.8%) | 111 (2.8%) | 1424 (35.9%) | |
| Current Smoking Status | ||||||
| No | 3033 (76.4%) | 1430 (79.3%) | 499 (79.5%) | 98 (89.1%) | 1006 (70.4%) | < 0.0001 |
| Yes | 936 (23.6%) | 373 (20.7%) | 129 (20.5%) | 12 (10.9%) | 422 (29.6%) | |
| Total | 3969 (100%) | 1803 (45.4%) | 628 (15.8%) | 110 (2.8%) | 1428 (36%) | |
| Circumcised | ||||||
| Not Circumcised | 2518 (63.4%) | 882 (48.9%) | 406 (64.5%) | 64 (57.7%) | 1166 (81.7%) | < 0.0001 |
| Circumcised | 1455 (36.6%) | 923 (51.1%) | 223 (35.5%) | 47 (42.3%) | 262 (18.3%) | |
| Total | 3973 (100%) | 1805 (45.4%) | 629 (15.8%) | 111 (2.8%) | 1428 (35.9%) | |
| Lifetime Number of Female Partners | ||||||
| 0 to 1 | 702 (17.7%) | 315 (17.5%) | 110 (17.5%) | 36 (32.4%) | 241 (16.9%) | < 0.0001 |
| 2 to 9 | 1582 (39.8%) | 644 (35.7%) | 166 (26.4%) | 53 (47.7%) | 719 (50.4%) | |
| 10 to 49 | 1253 (31.5%) | 633 (35.1%) | 241 (38.3%) | 13 (11.7%) | 366 (25.6%) | |
| ≥ 50 | 224 (5.6%) | 132 (7.3%) | 61 (9.7%) | 2 (1.8%) | 29 (2%) | |
| Refused | 212 (5.3%) | 81 (4.5%) | 51 (8.1%) | 7 (6.3%) | 73 (5.1%) | |
| Total | 3973 (100%) | 1805 (45.4%) | 629 (15.8%) | 111 (2.8%) | 1428 (35.9%) | |
| Number of Female Partners in Past 3 to 6 Months | ||||||
| None | 825 (20.8%) | 328 (18.2%) | 103 (16.4%) | 17 (15.3%) | 377 (26.4%) | < 0.0001 |
| 1 | 1615 (40.6%) | 809 (44.8%) | 215 (34.2%) | 54 (48.6%) | 537 (37.6%) | |
| 2 | 511 (12.9%) | 210 (11.6%) | 97 (15.4%) | 10 (9%) | 194 (13.6%) | |
| ≥ 3 | 518 (13%) | 259 (14.3%) | 116 (18.4%) | 11 (9.9%) | 132 (9.2%) | |
| Refused | 504 (12.7%) | 199 (11%) | 98 (15.6%) | 19 (17.1%) | 188 (13.2%) | |
| Total | 3973 (100%) | 1805 (45.4%) | 629 (15.8%) | 111 (2.8%) | 1428 (35.9%) | |
| Lifetime number of Male Partners | ||||||
| 0 to1 | 3557 (90.2%) | 1556 (87.2%) | 541 (86.8%) | 108 (97.3%) | 1352 (94.9%) | < 0.0001 |
| 2 to 9 | 228 (5.8%) | 127 (7.1%) | 49 (7.9%) | 3 (2.7%) | 49 (3.4%) | |
| ≥ 10 | 159 (4%) | 102 (5.7%) | 33 (5.3%) | 0 (0%) | 24 (1.7%) | |
| Total | 3944 (100%) | 1785 (45.3%) | 623 (15.8%) | 111 (2.8%) | 1425 (36.1%) | |
| Number of Male Partners in Past 3 to 6 Months | ||||||
| None | 3707 (93.9%) | 1638 (91.4%) | 581 (92.7%) | 110 (99.1%) | 1378 (97.2%) | < 0.0001 |
| ≥ 1 | 241 (6.1%) | 154 (8.6%) | 46 (7.3%) | 1 (0.9%) | 40 (2.8%) | |
| Total | 3948 (100%) | 1792 (45.4%) | 627 (15.9%) | 111 (2.8%) | 1418 (35.9%) | |
Exact Pearson chi-square p-value using the Monte Carlo estimation; bolded values denote statistically significant differences (p<0.05) across race groups.
RESULTS
Statistically significant differences were observed for the distribution of socio-demographic and sexual behavior characteristics across race (Table 1). Asian/PI men were younger, less likely to self-report as Hispanic ethnicity, attained a greater number of years of education, were more frequently married and never smokers, and reported the fewest female and male sexual partners. The twelve month incidence of any-, oncogenic-, and non-oncogenic HPV infection was significantly lower for Asian/PI race and highest for Black race (Table 2 and Figure 1). Incidence for oncogenic HPV types was 18.7% for Asians/PIs, 20.8% for multiple and mixed race, 25.1% for Whites, and 25.4% for Blacks (P < 0.001). Incidence of non-oncogenic types was 18.9% for Asians/PIs, 26.8% for multiple and mixed race, 31.4% for Whites, and 43.2% for Blacks (P < 0.001). Incidence for any HPV infection was 25.0% for Asians/PIs, 35.2% for multiple and mixed race, 37.3% for Whites, and 45.7% for Blacks (P < 0.001). Although Asian/PI men had the lowest rates of acquisition of new genital infections, once infected the duration of infection was similar to other races (Table 2).
Table 2.
Twelve-month incidence and median duration of HPV infection by race
| HPV Status | Total | White | Black | Asian/PI | Multiple and Mixed Race | P-value |
|---|---|---|---|---|---|---|
|
Twelve Month Incidence (95% Confidence Interval)1
|
||||||
| Any HPV | 37.3% (35.0%–39.8%) | 37.3% (33.9%–41.0%) | 45.7% (39.7%–52.3%) | 25.0% (16.1%–37.5%) | 35.2% (31.3%–39.3%) | < 0.0013 |
| Oncogenic | 23.5% (21.8%–25.3%) | 25.1% (22.6%–27.8%) | 25.4% (21.2%–30.2%) | 18.7% (11.4%–29.7%) | 20.8% (18.1%,–23.8%) | < 0.0013 |
| Non-oncogenic | 31.2% (29.2%–33.3%) | 31.4% (28.5%–34.6%) | 43.2% (37.8%–48.9%) | 18.9% (11.6%–30.1%) | 26.8% (23.6%–30.3%) | < 0.0013 |
|
Median Duration (95% Confidence Interval), months2
|
||||||
| Any HPV | 6.8 (6.6–7.0) | 6.8 (6.2–8.4) | 6.6 (6.1–7.6) | 6.7 (6.2–8.8) | 7.0 (6.2–10.0) | 0.2314 |
| Oncogenic | 6.6 (6.4–6.9) | 6.6 (6.1–8.1) | 6.5 (6.0–7.6) | 6.7 (6.2–12) | 6.7 (6.0–9.1) | 0.6834 |
| Non-oncogenic | 6.9 (6.6–7.2) | 6.9 (6.2–8.7) | 6.6 (6.1–7.8) | 6.5 (6.0–8.8) | 7.2 (6.2–11.7) | 0.0564 |
Estimate using the Kaplan-Meier method
Estimate using the clustered Kaplan-Meier method
p-value of the log-rank test for HPV incidence across entire follow-up period, by race group
p-value of the univariate Cox model with the robust covariance matrix estimator for HPV clearance across entire follow-up period, by race group
Figure 1.
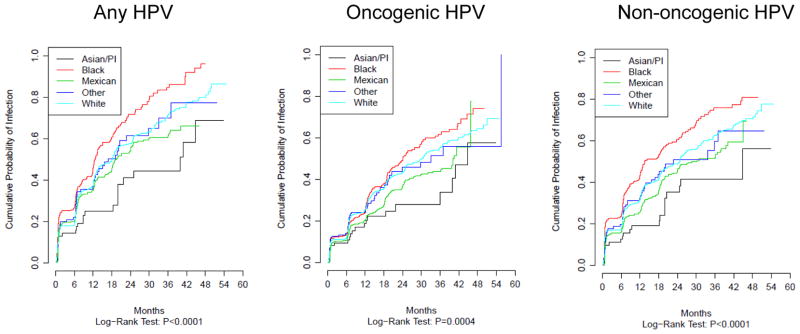
Incidence of HPV infection by race
In multivariable analyses Asian/PI race was associated with a lower probability of acquiring any-(HR=0.63; 95% CI 0.42–0.95) and non-oncogenic HPV infection (HR=0.61; 95% CI 0.40–0.93) when compared to Whites (HR=1.0) and marginally associated with oncogenic HPV (HR=0.72; 95% CI 0.46–1.13) (Table 3). There were no statistically significant differences for median duration of infection and clearance by race (Figure 2 and Table 3). Multiple and mixed race had a lower probability of acquiring non-oncogenic infections (HR=0.83; 95% CI 0.70–0.98) and borderline associations were observed for any- (HR=0.91; 95%CI 0.77–1.07) and oncogenic HPV (HR=0.92; 95% CI 0.79–1.09) (Table 3). Moreover, multiple and mixed race was significantly associated with a lower probability of clearing oncogenic infection (HR=0.89 95% CI 0.80–0.99), and a borderline significant associations were noted for any- (HR=0.96; 95% CI 0.89–1.04) and non-oncogenic infections (HR=0.98; 95% CI 0.90–1.07).
Table 3.
Risk of incidence and clearance of HPV by race
| HPV Status | Race | Incidence
|
Clearance
|
||
|---|---|---|---|---|---|
| Crude HR (95% CI) 1 | Multivariable HR (95% CI) 1 | Crude HR (95% CI) 1 | Multivariable HR (95% CI) 1 | ||
|
|
|
||||
| Any HPV | White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Black | 1.38 (1.16–1.65) | 1.15 (0.96–1.39)2 | 1.01 (0.79–1.29) | 1.02 (0.94–1.11)4 | |
| Asian/PI | 0.56 (0.38–0.84) | 0.63 (0.42–0.95)2 | 1.02 (0.94–1.11) | 1.03 (0.82–1.30)4 | |
| Multiple and Mixed Race | 0.87 (0.75–1.01) | 0.91 (0.77–1.07)2 | 0.93 (0.86–1.00) | 0.96 (0.89–1.04)4 | |
| Oncogenic | White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Black | 1.13 (0.95–1.35) | 1.03 (0.86–1.23)2 | 0.79 (0.56–1.10) | 0.96 (0.86–1.08)5 | |
| Asian/PI | 0.62 (0.41–0.96) | 0.72 (0.46–1.13)2 | 0.97 (0.87–1.08) | 0.80 (0.59–1.11)5 | |
| Multiple and Mixed Race | 0.80 (0.69–0.93) | 0.92 (0.79–1.09)2 | 0.89 (0.81–0.98) | 0.89 (0.80–0.99)5 | |
| Non–oncogenic | White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Black | 1.38 (1.17–1.63) | 1.13 (0.94–1.35)3 | 1.22 (0.90–1.66) | 1.06 (0.96–1.16)6 | |
| Asian/PI | 0.54 (0.36–0.83) | 0.61 (0.40–0.93)3 | 1.05 (0.95–1.16) | 1.25 (0.92–1.70)6 | |
| Multiple and Mixed Race | 0.83 (0.70–0.98) | 0.83 (0.70–0.98)3 | 0.95 (0.87–1.04) | 0.98 (0.90–1.07)6 | |
Hazard ratio (95% confidence interval)
Adjusted for age, marital status, total number of female partners, number of female partners in past 3 to 6 months, and number of male partners in past 3 to 6 months
Adjusted for age, marital status, circumcision, total number of female partners, total number of female partners in past 3 to 6 months, and total number of male partners.
Adjusted for HPV status at baseline, age, smoking status, number of female partners in past 3 to 6 months, and number of male partners in past 3 to 6 months
Adjusted for HPV status at baseline, age, and total number of female partners
Adjusted for HPV status at baseline, age, number of female partners in past 3 to 6 months, and total number of male partners in past 3 to 6
Figure 2.
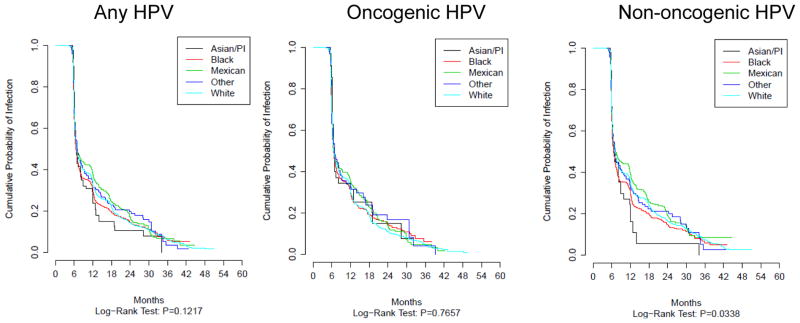
Clearance of HPV infection by race
To determine if sexual behavior influenced the observed associations, we explored a stratified analysis by number of lifetime female partners (Supplemental Table 1): 0 to 1, 2 to 9, and ≥ 10 partners. Because of sample size limitations there were few statistically significant results. However, generally Asian/PI race was associated with a lower probability of acquiring any-, oncogenic, and non-oncogenic infections across the three strata of lifetime number of female partners (e.g, for oncogenic infections; 0 partners: HR = 0.55 [95% CI 0.31 – 1.00]; 2 to 9 partners: HR = 0.64 [95% CI 0.33 – 1.21]; ≥ 10 partners: HR = 0.45 [95% CI 0.23 – 0.88]).
DISCUSSION
The purpose of this analysis is to assess the influence of self-reported race on acquisition (incidence) and persistence (clearance) of HPV infections among men participating in a multi-national prospective study. Overall we observed that self-reported Asian/PI race, compared to other self-reported races, was associated with a lower probability of acquiring an HPV infection, but there were no differences for duration of infection by race. Multiple and mixed race had the second lowest incidence of HPV and exhibited modest rates of new infections compared to the other races and in multivariable models multiple and mixed race was associated with a lower probability of acquiring an infection and with lower probability of clearing an infection.
Previous studies have reported a low prevalence of HPV infection among Asians in Continental Asia (17, 18) and we have shown racial differences in the prevalence of HPV [6] that persisted after adjustment for potential confounding and sexual behavior factors. Specifically, Asian/PI had the lowest HPV prevalence (42.2%) compared to Blacks (66.2%) and Whites (71.5%) and Asian/PI race was associated with a reduced risk HPV infection in multivariable analysis (prevalence ratio=0.65; 95% CI 0.52–0.80). The markedly reduced incidence of HPV among Asian/PI in the present analysis is consistent with the previously observed lower prevalence of HPV infection among Asian/PIs.
A possible explanation for the observed race-specific differences in HPV infection acquisition could be attributed to race-specific assortative mating. Assortative mating is a nonrandom pattern of mating where individuals with similar traits/phenotypes mate with one another more frequently than what would be expected under a random mating pattern [17–19]. Thus, if Asian/PI men in our study predominantly mated with Asian/PI the circulating pool of HPV among each group would be conceivably low. However, since HPV incidence among Asian/PI was relatively consistent when the data were stratified by lifetime sexual partners, differences in innate genetic variations that vary by race or intratypic HPV ancestral types may also explain our findings. Another possible explanation for the observed results in our current analyses could be attributed to potential geographic distribution of HPV types since rates of acquisition and clearance may vary by HPV types, as we do note slight variations in prevalence by country/study site (data not shown) for the most common disease causing HPVs (6, 11, 16, and 18).
Inherited genetic variations, namely single nucleotide polymorphisms (SNPs), in gene-disease associations can vary across ethnic and racial groups and there is evidence that such host genetic factors are also involved in HPV infection [20–23]. Infectious diseases exert selective pressure, and the host genes involved in the immune response are the most numerous and diverse in the human genome, indicating the evolutionary advantages of a varied immunological response to a wide range of infectious pathogens [24, 25]. The difference in prevalence and incidence of HPV by race in our cohort, which is not fully explained by environmental or behavioral factors, could be attributed to functional diversity of genetically-controlled factors including the immune response, inflammation, metabolism, and cell cycle, to name a few [24, 25]. Moreover, small candidate gene studies among women have provided evidence that host genetic factors are also involved in HPV infection but there are little data on differences by race. Interestingly, killer cell immunoglobin-like receptors and their human leucocyte antigen (HLA) ligands identify and destroy aberrant or virally infected cells are also highly polymorphic and vary in frequency across racial and ethnic populations [26]. Previous studies [27–29] have shown that HLA variants may exhibit a protective role against HPV infection among Asians. Although it is unclear if genetic variations across the racial groups are responsible for our observed race-specific associations, elucidating the possible role of germline variations in host genetics could have clinical relevance by identifying susceptible individuals for infection, clearance, or disease progression. With respect to intratypic HPV ancestral types, previous reports have suggested [30–32] that molecular intratypic variants of HPV subtypes have different geographic and racial distributions and that these intratypic variants appear to modulate risk of viral transmission, persistence and progression to clinically relevant lesions. Thus, potentially unmeasured intratypic variants in this cohort could explain our findings if a particular race is infected with a unique intratypic variant compared to the other racial groups.
There are many strengths and some limitations of this analysis that should be noted. First, the HIM Study is a unique resource since it is the only multi-national prospective study of the natural history of HPV infection in men and this is the first study to assess the incidence and clearance of HPV by race among men. Other strengths of this cohort study are the large sample size, the diversity in behavior and demographics, and study centers in three international cities. In this study we employed highly sensitive PCR methods for detection of 37 different HPV types. This method is not only highly sensitive and specific, but allows for relatively rapid evaluation of HPV infection in large epidemiological studies so that analyses can be completed in a timely manner. However, one of the limitations was the race-specific sample sizes. Specifically, Asian/PI race constitute only 2.8% of the study population while Whites made up 45.4% of the study population. Thus, we cannot totally rule out the possibility of selection bias in this study since Asian/PI race was under represented and about 80% of the Asian/PI population was from the United States site alone. The Mexican study site did not recruit any Asian/PI men and we acknowledge that the majority of men in the multiple and mixed race category were from Mexico. But, when we analyzed ‘Mexican race’ and ‘Other race’ categories separately, the estimates of effect for both race categories were quite similar for all analyses. Hence, the influence of multiple and mixed race on HPV acquisition and clearance was consistent in our analyses. We also acknowledge that because of collinearity between race and study location we cannot adjust for study location in our models. Another possible limitation is that race was self-reported and we do not possess data on ancestry informative markers to accurately classify our cohort. We also acknowledge that we cannot account for bias due to unmeasured or unknown confounding. Sexual behavior is potentially an important confounder in the association between race and HPV infection. We accounted for potential confounding by adjusting for sexual behavior and we conducted analyses stratified by number of female partners. However, residual confounding still may exist which could potentially inflate the observed point estimates. Of note, there was little evidence of confounding since results were generally consistent between the univariate and multivariable models. Finally, although the overall power of the study is robust, generalizability of the findings may be limited as participants were not randomly selected. Although there are numerous strengths and some limitations, our results should be interpreted with caution.
The mechanisms by which race influences HPV infection natural history among men is unknown. Overall, these results demonstrated that Asian/PI men had the lowest incidence of HPV and exhibited lower probability of acquiring new HPV infections. Multiple and mixed race men had the second lowest incidence of infection and however, while they had a lower probability of acquiring HPV, they also had a lower probability of clearing an HPV infection once acquired.
Supplementary Material
Acknowledgments
This project was supported through a grant from the National Cancer Institute, NIH, CA#RO1CA098803. The authors thank the following staff members for their dedication in recruiting, examining, and maintaining data on cohort participants, as well as conducting HPV DNA laboratory analyses: Kathy Eyring, CCRP; Christine Gage, ARNP; Nadia Lambermont, ARNP; Kim Isaacs, BA; Andrea M. Bobanic, BA;, Kayoko Kennedy, BA;, and the Tissue Core staff of the Moffitt Cancer Center for their help managing biological samples from the U.S. site; M Luiza Baggio, Roberto Silva, Lenice Galan, Elimar Gomes, Ricardo Cintra, Viviane Relvas, Filomena Cernicchiaro, Raquel Hessel, Sandra Araujo, Graça Ribeiro, Rosária Otero, Roberta Bocalon, Juliana Antunes, Rossana Terreri, Fernanda Silva, Rubens Matsuo, Ricardo Cunha, Vera Souza, Elisa Brito, Birgit Fietzek, from the Brazil site; Verónica Chávez, Aurelio Cruz, María Griselda Díaz, Rossana del Carmen González, Pilar Hernández, Ana Laura Landa, Alicia Rodríguez, and Oscar Rojas from the Mexico site. The authors also thank the Digene Corporation for kindly providing STM and collection vials at no charge to the study.
Footnotes
Conflicts of Interest: A. R. Giuliano receives support from Merck, is a member of the Merck Young Women’s Advisory Board, and serves on the speakers’ bureau for Merck. L. L. Villa is a consultant for Merck, Sharp, and Dohme.
References
- 1.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 2.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 3.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot HB, Morelock N. HPV in men is a women’s health issue. Nurs Womens Health. 2012;16:57–65. doi: 10.1111/j.1751-486X.2012.01701.x. [DOI] [PubMed] [Google Scholar]
- 5.HPV Vaccine - Questions & Answers. [ http://www.cdc.gov/vaccines/vpd-vac/hpv/vac-faqs]
- 6.Akogbe GO, Ajidahun A, Sirak B, Anic GM, Papenfuss MR, Fulp WJ, et al. Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States. Int J Cancer. 2012;131:E282–291. doi: 10.1002/ijc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–476. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 8.Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, et al. Human papillomavirus infection in Shanxi Province, People’s Republic of China: a population-based study. Br J Cancer. 2006;95:96–101. doi: 10.1038/sj.bjc.6603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliano AR, Lazcano E, Villa LL, Flores R, Salmeron J, Lee JH, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–1257. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prevent. 2008;17:2036–2043. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores R, Abalos AT, Nielson CM, Abrahamsen M, Harris RB, Giuliano AR. Reliability of sample collection and laboratory testing for HPV detection in men. J Virol Methods. 2008;149:136–143. doi: 10.1016/j.jviromet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–3027. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 15.Ying Z, Wei LJ. The Kaplan-Meier estimate for dependent failure time observations. J Multivar Anal. 1994;50:17–29. [Google Scholar]
- 16.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 17.Burrell AS, Disotell TR. Panmixia postponed: ancestry-related assortative mating in contemporary human populations. Genome Biol. 2009;10:245. doi: 10.1186/gb-2009-10-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev Camb Philos Soc. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- 19.Godoy R, Eisenberg DTA, Reyes-Garcia V, Huanca T, Leonard WR, McDade TW, et al. Assortative mating and offspring well-being: theory and empirical findings from a native Amazonian society in Bolivia. Evol Hum Behav. 2008;29:201–210. [Google Scholar]
- 20.Chattopadhyay K. A comprehensive review on host genetic susceptibility to human papillomavirus infection and progression to cervical cancer. Indian J Hum Genet. 2011;17:132–144. doi: 10.4103/0971-6866.92087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr. 2003:35–40. doi: 10.1093/oxfordjournals.jncimonographs.a003480. [DOI] [PubMed] [Google Scholar]
- 22.Wang SS, Gonzalez P, Yu K, Porras C, Li Q, Safaeian M, et al. Common genetic variants and risk for HPV persistence and progression to cervical cancer. PLoS One. 2010;5:e8667. doi: 10.1371/journal.pone.0008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill AV. The genomics and genetics of human infectious disease susceptibility. Annu Rev Genomics Hum Genet. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. [DOI] [PubMed] [Google Scholar]
- 24.Hill AV. Immunogenetics and genomics. Lancet. 2001;357:2037–2041. doi: 10.1016/S0140-6736(00)05117-5. [DOI] [PubMed] [Google Scholar]
- 25.Burgner D, Jamieson SE, Blackwell JM. Genetic susceptibility to infectious diseases: big is beautiful, but will bigger be even better? Lancet Infect Dis. 2006;6:653–663. doi: 10.1016/S1473-3099(06)70601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010;129:8–19. doi: 10.1111/j.1365-2567.2009.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bontkes HJ, van Duin M, de Gruijl TD, Duggan-Keen MF, Walboomers JM, Stukart MJ, et al. HPV 16 infection and progression of cervical intra-epithelial neoplasia: analysis of HLA polymorphism and HPV 16 E6 sequence variants. Int J Cancer. 1998;78:166–171. doi: 10.1002/(sici)1097-0215(19981005)78:2<166::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Chan PK, Cheung JL, Cheung TH, Lin CK, Siu SS, Yu MM, et al. HLA-DQB1 polymorphisms and risk for cervical cancer: a case-control study in a southern Chinese population. Gynecol Oncol. 2007;105:736–741. doi: 10.1016/j.ygyno.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Chan PK, Cheung JL, Cheung TH, Lin CK, Tam AO, Chan DP, et al. HLA-B alleles, high-risk HPV infection and risk for cervical neoplasia in southern Chinese women. Int J Cancer. 2006;118:1430–1435. doi: 10.1002/ijc.21528. [DOI] [PubMed] [Google Scholar]
- 30.Junes-Gill K, Sichero L, Maciag PC, Mello W, Noronha V, Villa LL. Human papillomavirus type 16 variants in cervical cancer from an admixtured population in Brazil. J Med Virol. 2008;80:1639–1645. doi: 10.1002/jmv.21238. [DOI] [PubMed] [Google Scholar]
- 31.Amador-Molina A, Gonzalez-Montoya JL, Garcia-Carranca A, Mohar A, Lizano M. Intratypic changes of the E1 gene and the long control region affect ori function of human papillomavirus type 18 variants. J Gen Virol. 2013;94:393–402. doi: 10.1099/vir.0.045807-0. [DOI] [PubMed] [Google Scholar]
- 32.Sichero L, Villa LL. Epidemiological and functional implications of molecular variants of human papillomavirus. Braz J Med Biol Res. 2006;39:707–717. doi: 10.1590/s0100-879x2006000600002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


